Translate this page into:
Intra-Abdominal Pressure Monitoring in Acute Severe Pancreatitis—A Boon or Bane?
*Corresponding author: Niyas Ahamed, MS, FAIS, FMAS, No 3, Meenakshi Avenue, Kalainagar 1st street, Madurai, Tamil Nadu, 625017, India. niyasdr@yahoo.co.in
Abstract
Background
Intra-abdominal hypertension (IAH) is increasingly reported in patients with acute pancreatitis, and is caused by visceral edema, massive fluid resuscitation, paralytic ileus, and retroperitoneal inflammation. Patients with acute severe pancreatitis actually suffer from abdominal compartment syndrome (ACS)/IAH and since there is a strong correlation between early organ dysfunction and mortality in these patients, IAH appears to be an active and attractive target for early analysis and intervention.[1]
Aim
The study is undertaken to estimate the significance of intra-abdominal pressure monitoring in acute severe pancreatitis.
Objectives
The objective of this study is to evaluate relationship between intra-abdominal pressure (IAP) and severity of acute pancreatitis and measure outcome in the form of intensive care unit (ICU) stay, hospital stay, treatment modality, and condition on discharge.
Methodology
A total of 50 patients diagnosed as acute severe pancreatitis were enrolled in this observational study. IAP monitoring was started on admission, once after controlling pain and then every 4 hours. IAP was measured via transvesical route. Data were collected on the length of the hospital stay, the development of systemic inflammatory response syndrome (SIRS), multiorgan failure, the extent of necrosis, the presence of infection, and mortality.
Results
IAH was present in 86% of patients with acute severe pancreatitis, which shows IAP monitoring is essential in managing these patients. Severity estimation by IAP monitoring is consistent with alternative laboratory parameters like Ranson’s score (p = 0.002), SIRS (p = 0.013), organ failure/multiple organ dysfunction syndrome (p = 0.009). Two deaths were incurred during the study period.
Conclusions
IAP measurement in acute severe pancreatitis is a cost-effective and prognostic marker. Timely diagnosis and management of IAH/ACS through IAP monitoring can prevent major comorbidity (ICU/hospital stay) and mortality.
Keywords
IAP
Severe acute pancreatitis
Outcome
Introduction
Although intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are considered due to problems occurring in surgical patients, they have been identified as a cause of organ dysfunction in patients without apparent abdominal surgical condition like acute pancreatitis.[1] Acute pancreatitis is a common parlance in the surgical emergency ward, accounting for a major fraction of patients presenting with abdomen pain. It is classified into mild, moderate, and severe categories by the Revised Atlanta Guidelines.[2] While mild and moderate varieties form the major bulk of cases, severe acute pancreatitis constitutes the most morbid and mortal subtype.
Severe acute pancreatitis is defined clinically by the presence of C-reactive protein (CRP) >150 mg/dL, Ranson’s score >3 on admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score >8, and computed tomography (CT) severity index >7.[2] Complications of severe acute pancreatitis include pancreatic necrosis, infections, organ failure, sepsis, and multiple organ dysfunction syndrome (MODS).
Though newer molecular markers for predicting disease severity offer hope, they provide little by means as a point of intervention.
Raised intra-abdominal pressure (IAP) is a feature of acute pancreatitis due to visceral edema, increased fluid resuscitation. Although mild and moderate varieties of acute pancreatitis produce only a transient and mild increase in IAP, it is the severe variety that results in IAH. As per the newer definitions set by the World Society on the Abdominal Compartment Syndrome (WSACS),[3] IAH is defined as a sustained increase in IAP >12 mm Hg and ACS is a sustained increase in IAP >20 mm Hg.[4,5] IAH may prove detrimental in hospitalized patients leading to ACS.
The physiological insults following IAH are decreased venous return, decreased cardiac output, hypotension, decreased urine output, metabolic acidosis, and shock.
Despite having many methods of monitoring IAP, using a central venous pressure (CVP) manometer is a cost-effective method of determining IAP through transvesical route [Figure 1]. As per the recent guidelines on IAP monitoring, instillation of larger volumes (50–300 mL) of saline for IAP monitoring is not necessary as it may lead to falsely elevated values.[6]
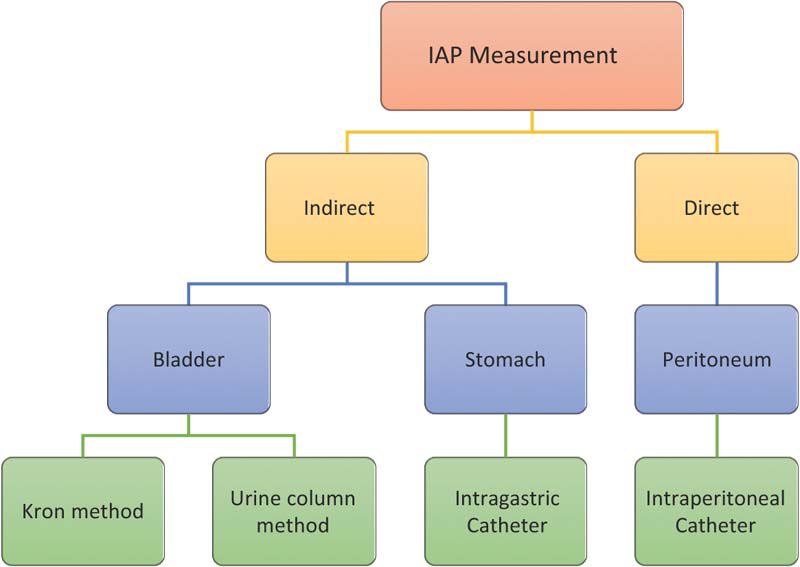
- Different methods of intra-abdominal pressure (IAP) measurement.
In literature reviews, patients undergoing abdominal decompression for acute severe pancreatitis have shown excellent results with respect to outcome which suggests that IAP may be considered a target for early intervention.[7] Thus, there is a need for a study showing that a rise in IAP results in deterioration of patient’s condition and significantly affects outcome. Also, there is need of study to show that IAP can be a helpful prognostic tool.
Routine IAP monitoring in such patients with severe variety may give an insight into prompt management in preventing the dire effects of ACS [Figure 2].[8]
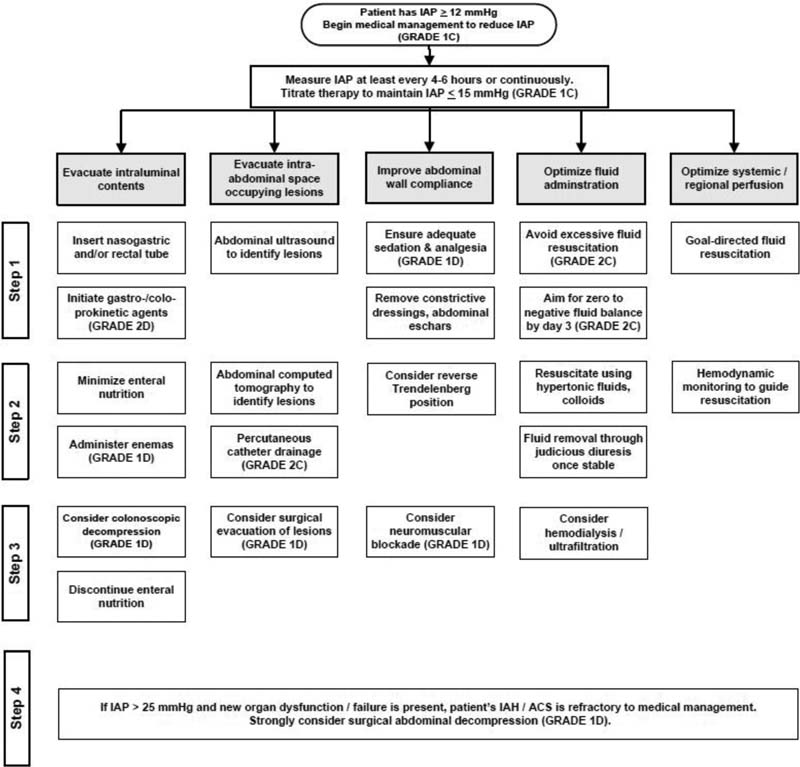
- Algorithm for raised intra-abdominal pressure (IAP) management. ACS, abdominal compartment syndrome; IAH, intra-abdominal hypertension.
This study aims at estimating the significance of IAP monitoring in determining the severity of acute pancreatitis and its prognostic value in acute severe pancreatitis.
Methodology
This observational study was undertaken in Government Rajaji Hospital, Madurai, for a period of 1 year from November 2019 to November 2020. A total of 50 patients attending the general surgery emergency and outpatient department services, who were diagnosed with severe acute pancreatitis, were studied after obtaining informed consent from them.
Inclusion criteria
-
Patients > 18 years and < 60 years of age in both sexes.
-
Patients with duration of pancreatitis less than 72 hours.
Exclusion criteria
-
Patients <18 years and >60 years of age.
-
Pregnant females.
-
Patients with duration of pancreatitis >72 hours.
-
Patients with severe comorbidities (uncontrolled diabetes mellitus, uncontrolled hypertension, advanced malignancies, severe renal, cardiac and liver dysfunction)
-
Patient not consented for inclusion in the study.
Ethical clearance
Obtained
Methods of study
On admission, the distinction between nonsevere and severe acute pancreatitis decided on the basis of Ranson’s score (at admission> 3) or CRP > 150 mg/dL. For the purpose of standardizing data, the first hospital day should be designated as day 1.
Using a Foley catheter (16-F or 18-F), an intravenous infusion set, a 50-mL syringe, a CVP monitor, and a hemostat, provides a low-cost assessment of the IAP [Figure 3]. To determine IAP, a Foley catheter is inserted into the bladder and instilled with 25 mL distilled water and measured using CVP monitor. IAP is measured on admission after control of severe acute pain by optimal use of analgesics including tramadol, morphine, pethidine, and fentanyl to minimize the confounding effect of pain on IAP measurement. Further IAP measurements will be done every 8 hours on first day. Diagnosis of IAH is done by a fixed protocol as discussed below. IAP measurements are done till Foley catheter is required in situ for patients. IAP measurements are done every 4 hours for those patients with ACS.

- Foley catheter/central venous pressure monitor for intra-abdominal pressure measurement.
“Maximum IAP” is defined as the maximum pressure recorded in all readings and “Mean IAP” is defined as the mean of all pressure values recorded on first day. IAH is defined by a sustained or repeated pathologic elevation of IAP≥12 mm Hg (WSACS). In this study, we define the presence of IAH as consistently increased IAP ≥ 12 mm Hg as recorded by the first three readings taken during at least 8 hours. Early severe pancreatitis mostly determines the outcome. IAH is classified into five groups.
Class A/Grade 0 = no IAH
Class B/Grade I = 12–15 mm Hg,
Class C/Grade II = 16–20 mm Hg,
Class D/Grade III = 21–25 mm Hg,
Class E/Grade IV ≥ 25mm Hg.
Class E is equivalent to ACS. Each patient is categorized as above and are followed up for a period of 45 days. For each class, vitals of day 1, CRP, systemic inflammatory response syndrome (SIRS) status, Ranson’s score, presence of any complications, or organ failure are noted, apart from standard demographic and clinical data. All patients are treated by standard management of pancreatitis protocol based on Revised Atlanta Guidelines. The data will be analyzed statistically.
Analytical Tools
Data collected include the demographic data, CT severity index, Ranson’s score, SIRS, organ failure, intensive care unit (ICU) stay, hospital stay, mortality, and are analyzed with Chi-square test, p-value using SPSS software.
Results
After analyzing the study results of 50 participants that included 34 men and 16 women, the following results were arrived at. The mean age was 43.02 years.
The most predominant etiological causes of acute pancreatitis were alcoholism (62%) followed by biliary (26%) pathology [Figure 4].
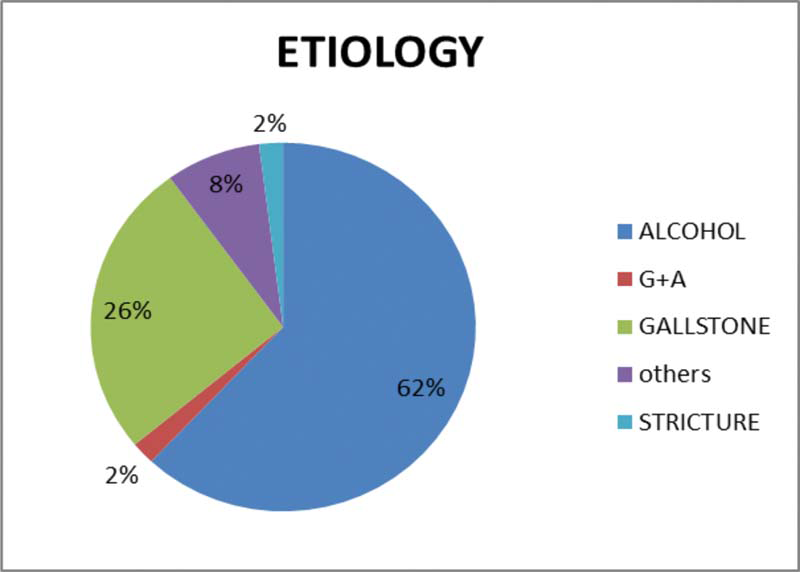
- Etiology for acute pancreatitis.
The recorded mean IAP correlated significantly with various severity scores like SIRS (p = 0.013), Ranson’s score (p = 0.002), organ failure/MODS (p = 0.009), CT severity index (p = 0.016), hospital stay (p = 0.001); however, mean IAP did not significantly correlate with serum amylase (p = 0.394).
Maximal IAP in most of patients was documented on day 1 of admission.
No IAH was noted in 7 patients (14%), mild IAH (class B) noted in 18 (36%), moderate IAH (class C) noted in 16 (32%), severe IAH (class D) noted in 8 (16%), and ACS noted in 1 patient (2%) [Figure 5].

- Distribution of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) in pancreatitis.
In 27 patients, no organ dysfunction was noted; 13 patients had single organ failure (renal/hepatic/respiratory) and 10 patients had MODS. Only one patient had pancreatic necrosis belonging to class C, and was treated conservatively. However, five patients with no IAH had SIRS, only one had one organ failure and none had MODS. Two (4%) deaths were seen, one belonging to class D (severe IAH) and other patient belonged to class E (ACS). Both patients had MODS. Thirty-six patients (72%) were cured of acute pancreatitis and 12 patients (24%) improved clinically at the time of discharge. On the 45th day of follow-up, 84% of patients had been totally cured [Table 1].
Complication/outcome |
Class of IAH |
|||||
A |
B |
C |
D |
E |
||
Patients |
7 |
18 |
16 |
8 |
1 |
|
Total Ranson’s score |
4.7 |
4.8 |
5.3 |
6.6 |
9 |
|
CT severity index |
Mild |
6 |
16 |
7 |
0 |
0 |
Moderate |
1 |
2 |
8 |
1 |
0 |
|
Severe |
0 |
0 |
1 |
7 |
1 |
|
Cured |
7 |
16 |
12 |
1 |
0 |
|
Improved |
0 |
2 |
4 |
6 |
0 |
|
Death |
0 |
0 |
0 |
1 |
1 |
|
SIRS |
5 |
8 |
16 |
7 |
1 |
|
One organ failure |
1 |
5 |
5 |
2 |
0 |
|
MODS |
0 |
0 |
3 |
6 |
1 |
|
No organ failure |
6 |
13 |
8 |
0 |
0 |
|
Hospital stay (days) |
5.8 |
8.9 |
7.8 |
19.3 |
30 |
|
Abbreviations: CT, computed tomography; IAH, intra-abdominal hypertension; MODS, multiple organ dysfunction syndrome; SIRS, systemic inflammatory response syndrome.
The administration of prokinetic motility agents such as erythromycin, metoclopramide, or neostigmine appeared to hold promise in evacuating the intraluminal contents. Moreover, sedation and muscle relaxant (only for mechanical ventilated patients) were also used properly to lower IAP.
Discussion
Acute pancreatitis is a significant differential diagnosis of abdomen pain; its severity lures to be a considerable problem with respect to its diagnosis and management.
Critically ill patients with acute pancreatitis have high IAP that correlates with the degree of organ dysfunction and length of intensive care.[9] Early diagnosis of this complication may pave way for a timely intervention, be it medical or surgical, which ultimately survives the patient.
For patients with severe acute pancreatitis, IAH could be especially deleterious because increased IAP results in general splanchnic hypoperfusion, decreased blood flow to pancreas and together with bacterial translocation may predispose the patient to infected necrosis and poor outcome.[10]
In selected trauma and surgical patients, with severe IAH, as shown in literature studies, the risk of organ dysfunction can be minimized considerably with a timely decompressive laparotomy, especially in those patients who are unresponsive to nonoperative management. The same effect can be extrapolated to patients with severe acute pancreatitis.[11–13]
Since IAH being an early phenomenon, it is amenable to intervention that makes more sense to recommend its surveillance[14] as done in this study.
Severe disease has long been managed as a homogenous group. Our study points at possible subcategories among the array of patients with severe disease as discriminated by their IAP values.[15]
This study is cornered down to identify the cost-effective assessment of IAH that is repeatable and accurate. In that means, using a CVP manometer with a Foley catheter provides a better alternative to other laboratory analysis and IAP measuring techniques. The problem of instillation of >50 mL of normal saline in measuring IAP leading to false positive results has been minimized in this study by using only 25 mL of saline.
Measurement of IAP should not be performed until pain control has been achieved.
Day 1 of admission recorded the maximum IAP in majority of the study population, the results of which are similar to that of Keskinen et al.[9]
In our study, 86% of patients presented with IAH. The mortality assessment with these patients correlated to the IAP measurement values. The morbidity with respect to hospital stay was directly proportional to the increase in IAP.
This study analysis in comparison to other literature reviews provided similar or better results with respect to identifying the severity and predicting the outcome of the patients presenting with severe acute pancreatitis.
Routine transvesical IAP pressure measurements in all patients with acute pancreatitis may not be necessary but only patients who manifest with Ranson’s score > 3/CRP >150 mg/dL/organ failure/persistent SIRS should be offered IAP surveillance.
Conclusion
What is known?
-
-
Acute severe pancreatitis may lead to SIRS/MODS.
-
-
Markers of acute severe pancreatitis like Ranson’s score, APACHE II, CRP, Bedside Index for Severity in Acute Pancreatitis (BISAP), CT severity index, etc.,
-
-
Elevated IAP in acute severe pancreatitis.
What is new?
-
-
Early IAP measurement significantly determines SIRS/MODS/ICU and hospital stay.
-
-
IAH produces increased morbidity and mortality in severe pancreatitis patients.
-
-
IAP measurement transvesically is easy, minimally invasive, prognostic modality, and a cheaper alternative.
-
-
Early diagnosis and prompt intervention of IAH can prevent major catastrophe.
Conflict of interest
I (we) certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- Abdominal compartment syndrome in severe acute pancreatitis-when to decompress? Eur J Trauma Emerg Surg. 2008;34:11-6.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-11.
- [CrossRef] [PubMed] [Google Scholar]
- Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-32.
- [CrossRef] [PubMed] [Google Scholar]
- Moderate increase in intraabdominal pressure attenuates gastric mucosal oxygen saturation in patients undergoing laparoscopy. Anesthesiology. 2004;100:1081-7.
- [CrossRef] [PubMed] [Google Scholar]
- Is it wise not to think about intraabdominal hypertension in the ICU? Curr Opin Crit Care. 2004;10:132-45.
- [CrossRef] [PubMed] [Google Scholar]
- Rational intraabdominal pressure monitoring: how to do it? Acta Clin Belg. 2007;62():16-25.
- [PubMed] [Google Scholar]
- Acute pancreatitis In: O’Donnell JM, Nácul FE, eds. Surgical Intensive Care Medicine. Boston, MA: Springer; 2010.
- [CrossRef] [Google Scholar]
- Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190-206.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intra-abdominal pressure in severe acute pancreatitis. World J Emerg Surg. 2007;2:2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care. 2005;11:156-71.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747-53.
- [CrossRef] [PubMed] [Google Scholar]
- Temporary abdominal closure: a prospective evaluation of its effects on renal and respiratory physiology. J Trauma. 1998;45:914-21.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective characterization and selective management of the abdominal compartment syndrome. Am J Surg. 1997;174:667-72. discussion 672–3
- [CrossRef] [PubMed] [Google Scholar]
- Intra-abdominal pressure as a marker of severity in acute pancreatitis. Surgery. 2007;141:173-8.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-abdominal pressure in the early phase of severe acute pancreatitis: canary in a coal mine? Results from a rigorous validation protocol. Gut Liver. 2013;7:731-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







