Translate this page into:
A Journey from Schizophrenia to Paraneoplastic Limbic Encephalitis

*Corresponding author: Dr Rajalaxmi Satapathy, Associate professor, Department of Neurology, Kalinga Institute of Medical Sciences, Kalinga Institute of Industrial Technology University, Bhubaneswar, Odisha, India. satapathyrajalaxmi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Satapathy R, Behuria P. A Journey from Schizophrenia to Paraneoplastic Limbic Encephalitis. Int J Recent Sur Med Sci. 2023;9:127-9. doi: 10.25259/IJRSMS-2022-8-1
Abstract
A 58-year female presented with behavioral changes, dementia, and hallucinations for which she was initially treated as a case of schizophrenia. Later she developed hypersomnolence, speech difficulty and urinary incontinence. She also developed seizures later. On investigations cerebrospinal fluid (CSF) cell count was high and chest x-ray revealed homogeneous opacity over the left upper and midzone. Contrast-enhanced computerized tomography (CECT) Thorax revealed apical segment collapse consolidation with air bronchogram and bilateral pleural effusion. Multiple hepatic and bony metastasis s/o primary bronchoalveolar carcinoma. Osteolytic lesions were also found on skull x-ray and on pelvis x-ray. Tissue diagnosis couldn’t be done as the patient’s general condition deteriorated and the patient left against medical advice. From the above clinical picture and investigation findings, it was diagnosed as a case of paraneoplastic limbic encephalitis.
Keywords
Bronchoalveolar carcinoma
Hallucination
Metastasis
Introduction
Paraneoplastic limbic encephalitis associated with an underlying malignancy is relatively rare and autoimmune in nature.[1] It commonly involves the medial temporal lobe. The common clinical features include impaired memory, behavioral changes, seizures, irritability, hallucinations, altered sensorium or focal deficits in the form of paresis of the extremities.[2] We are reporting a case that was treated as a psychiatric disorder initially but was later found to have bronchogenic cancer.
Case Report
A 58-year-old female presented with behavioral changes for five months. She developed socially inappropriate behaviors like undressing in front of others, inappropriate crying and laughter, delusion and visual hallucinations. She also developed pain in multiple parts of the body, for which she was evaluated in detail. The patient was diagnosed with schizophrenia and was treated with olanzapine and aripiprazole. But no improvement was seen. The patient was admitted to the neurology ward one month later with speech difficulty and an altered sleep pattern. She had increased sleep and daytime hypersomnolence. She gradually stopped interacting with others. She often got up from sleep or screamed out of dreams at midnight in the last month. She also developed urinary incontinence. During her hospitalization, she developed two episodes of generalized tonic clonic convulsions. She was known to be hypertensive. She was treated with antipsychotics, antiepileptics and antihypertensives. The patient was disoriented with indistinct speech. The plantar was bilateral flexor. On auscultation of the chest, there were diminished breath sounds in the left infrascapular area. Other system examinations were normal. All routine investigations, complete blood count (CBC), erythrocyte sedimentation rate (ESR), viral markers, antinuclear antibody (ANA) Profile, thyroid stimulating hormone (TSH), Serum M band, venereal disease research laboratory (VDRL), s vit B12 and anti thyroid peroxidase (TPO) antibody, were normal. The cerebrospinal fluid (CSF) study revealed a total cell count of 8, with all mononuclear cells, no malignant cells. Resting sugar, protein and adenosine deaminase (ADA) in CSF were normal. The autoimmune encephalitis panel and the viral encephalitis panel were negative. The electroencephalogram (EEG) showed diffuse slowing. Magnetic resonance imaging (MRI) Brain showed only periventricular hyperintensity. The chest X-ray showed homogeneous opacity over the left upper and midzones [Figure 1]. Contrast-enhanced computerized tomography (CECT) Thorax revealed apical segment collapse consolidation with air bronchogram, bilateral pleural effusion. Multiple hepatic and bony metastasis s/o primary bronchoalveolar carcinoma [Figure 2]. Osteolytic lesions were also found on the skull X-ray [Figure 3] and pelvis X-ray [Figure 4]. Tissue diagnosis couldn’t be done as the patient’s general condition deteriorated, and the patient left against medical advice.
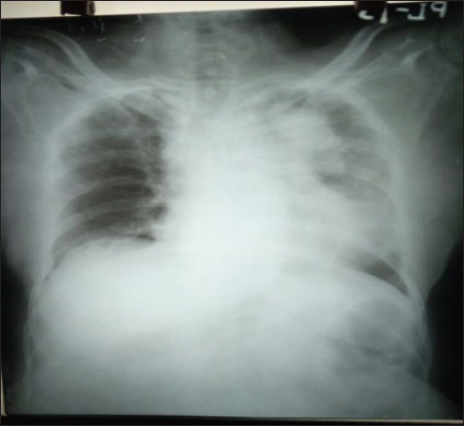
- Chest x-ray showed homogeneous opacity over left upper and midzone.

- Contrast-enhanced computerized tomography (CECT) Thorax revealed apical segment collapse consolidation (blue arrow).
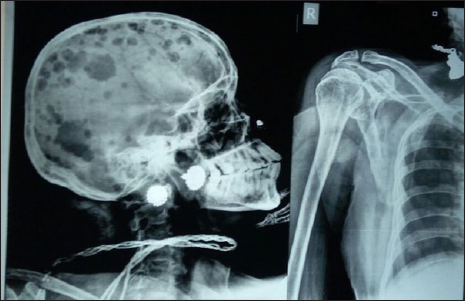
- Osteolytic lesions also found on skull x-ray and x-ray of shoulder.

- Osteolytic lesion on x-ray pelvis.
Discussion
In our case, the patient presented with subacute encephalopathy. The differential diagnosis includes Hashimoto encephalopathy, autoimmune encephalitis, Creutzfeldt Jakob disease and paraneoplastic limbic encephalitis. We excluded them one by one through different investigations. MRI Brain was almost normal. As the patient was an elderly female, paraneoplastic encephalitis is a possibility. Paraneoplastic limbic encephalitis is a rare disorder that coexists with 50% of small cell lung cancers, usually immune mediated.[3] Many cases of lung cancer present with new onset seizures due to paraneoplastic limbic encephalitis.[4] Usually, these patients have anti-Hu antibody positive.[5] Limbic encephalitis is a subacute encephalopathy characterized by impaired memory, personality changes and sleep disturbances.[6] Treatment is usually symptomatic after the removal of primary tumor.[7]
Conclusion
The boundary between neurology and psychiatry is becoming increasingly blurred. So, the job of a neurologist is to rule out any chance of an organic disorder before stamping it as psychiatric disorder. Paraneoplastic encephalitis must be included in the differential diagnosis of any elderly patient presenting with encephalopathy.
Acknowledgment
None.
Ethical Approval
The research/study complied with the Helsinki Declaration of 1964.
Declaration of patients consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- et al. Paraneoplastic limbic encephalitis associated with lung cancer. Sci Rep. 2018;8:6792.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Paraneoplastic Limbic Encephalitis. [Updated 2021 Jun 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519523/ [Last accessed on 2022 Aug 3]
- [Google Scholar]
- Paraneoplastic limbic encephalitis in a patient with extensive disease small-cell lung cancer. Mol Clin Oncol. 2017;6:575-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Paraneoplastic limbic encephalitis as a cause of new onset of seizures in a patient with non-small cell lung carcinoma: a case report. J Med Case Reports. 2008;2:270.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Limbic encephalitis and small cell lung cancer, clinical and immunological features. Brain. 1997;120:923-8.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic limbic encephalitis. Clinicopathological correlations. J Neurol Neurosurg Psychiatry. 1990;53:1084-88.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Limbic encephalopathy as a non-metastatic complication of oat cell lung cancer. Am J Med. 1983;75:518-20.
- [CrossRef] [PubMed] [Google Scholar]







