Translate this page into:
A Study to Assess the Burden of Hematological Malignancies at a Tertiary Care Center

Corresponding author: Dr. Alhad Mulkalwar, Department of Medicine, Seth Gordhandas Sunderdas Medical College & King Edward Memorial Hospital, Parel, Mumbai, Maharashtra, India. alhad.mulkalwar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pai V, Gite P, Joshi K, Mulkalwar A, Kate V. A Study to Assess the Burden of Hematological Malignancies at a Tertiary Care Center. Int J Recent Surg Med Sci. 2024;10:51-56. doi: 10.25259/IJRSMS_13_2023
Abstract
Objectives
Hematological malignancies place a significant financial and medical burden on the affected patients. Well-equipped oncology centers providing healthcare for these patients are also sparse. By studying the epidemiology of those diagnosed with hematological malignancies, it can be correlated with the outcomes and compared with global and national trends. This will allow healthcare decision-makers at all levels to make informed decisions, while allocating resources to combat hematological malignancies. This study is aimed at understanding the pattern of various hematological malignancies presenting at a tertiary care center including their clinical profile, risk factors, etiology and presentation.
Material and Methods
This was an observational prospective study conducted in King Edward Memorial Hospital, Mumbai, India. Patients aged 12 years and above suffering from hematological malignancies were recruited from the medical wards. The history and systemic examination findings were recorded from patients’ records. The patients were treated as per the discretion of the treating physician and all investigations advised by the treating physician were noted in the respective case record forms.
Results
The maximum cases were from the age group 36–50 years, i.e., 32 in number (40%), followed by the age group 51–65 years (25%). A greater number of male patients were encountered than female ones (67.5% vs 32.5%). Most of the patients were unskilled laborers followed by semi-skilled workers. Pesticide exposure was the commonest etiological factor reported in 16 (20%) patients, followed by exposure to silica that was reported in nine patients (11.25%).
Conclusion
The common age group to encounter hematological malignancies in patients is 36–50 years, being more prevalent among males. History of exposure to risk factors such as pesticides is commonly seen. Factors such as age and family history do not correlate with the outcome. Females tend to have a better outcome than males.
Keywords
Lymphoma
Leukemia
Pesticides
Hematolymphoid neoplasms
INTRODUCTION
Hematological malignancies are primary cancers of the blood and blood-forming organs, i.e., the bone marrow and lymphoid tissues. They are usually clonal in origin and are very often accompanied with chromosomal abnormalities.[1] These malignancies are caused by mutations or genetic insult in somatic cells, which can result from environmental agents such as viruses, ionizing radiation, and chemicals. The manner in which various cancers manifest clinically varies and is primarily determined by the type of illness and how severe it is.
Hematological malignancies are a group of heterogeneous mitotic disorders that all arise from bone marrow and lymphatic system cells.[2] When anything goes wrong in the control of the division or lifespan of a blood cell or its precursor, a hematological malignancy results.[3] It is characterized by the wide, accelerated and disordered proliferation of leukocytes and their progenitors as well as the presence of immature leukocytes, frequently in extremely high concentrations, in the blood. One of the most frequently occurring cancers in all races or ethnicities is leukemia, with a relative proportion varying between 25% and 40%.[4] In 2013, more than 57% of the new cases of leukemia were found to be males.[5] Exposure to environmental and occupational carcinogens is a known result in various types of leukemias in males.[6,7] In the last few decades, epidemiology has played a vital part in learning about the causes of different types of leukemias. The burden of cancer, including Hematological malignancies, is greater in developing countries due to growing population, urbanization, aging, a change in dietary habits, better control of infections and an increase in the consumption of tobacco.
Hematological malignancies consist of a collection of heterogeneous malignant conditions that all originate from cells of the bone marrow and the lymphatic system.[8] They are derived from a single cell in the marrow or peripheral lymphoid tissue which has undergone genetic alteration.[9] The three major groups of hematological malignancies are: leukemias, lymphomas, and plasma cell neoplasms, with the other hematological malignancies being polycythaemia vera, myelodysplastic syndrome, and primary myelofibrosis.
Hematological malignancies make up 20% of the diagnosis of cancer, a leading cause of death globally.[10] While complete cure of hematological malignancies can be achieved with the advancements in therapy, around 70% of patients become critical while admitted to hospital.[11,12] The risk of complications has also been increased with treatment modalities like intensive chemotherapy and stem cell transplant.[13] Leukemia is a group of cancers that usually begin in the bone marrow and results in high numbers of underdeveloped white blood cells called “blasts”. In 2012, 352,000 people developed leukemia, resulting in 265,000 deaths. In children, it is the most common type of cancer, with three-quarters of leukemia cases in children being Acute lymphocytic leukemia (ALL). However, about 90% of all leukemia are diagnosed in adults, with Acute myeloid leukemia (AML) and Chronic lymphocytic leukemia (CLL) being most common.
Hematological malignancies are a major medical and financial burden to the affected patients. Though the pattern of distribution of these malignancies in different parts of the globe has been studied, not much is known about their prevalence and distribution in our environment. A thorough study of the epidemiology of these cancers may help identify the environmental risk factors and give an epidemiological framework for the development of preventative measures intended to lower the prevalence of these cancers. This study aimed at understanding the pattern of various hematological malignancies presenting at a tertiary care center including their clinical profile, risk factors, etiology and presentation.
MATERIAL AND METHODS
This was an observational prospective study conducted over a period of around two years from December 2015 to November 2017 in King Edward Memorial Hospital, Mumbai, India. Approval from the Institutional Ethics Committee was obtained before the commencement of the study, approval reference number IEC/209/2015. Patients aged 12 years and above suffering from a hematological malignancy were recruited from the medical wards after explaining in detail the nature of the study and obtaining written informed consent for the same. The history and systemic examination findings were recorded from patients’ admission records. Parameters that were noted in the case record form include demographic details including age and gender, occupation, nature of work and occupational exposure, clinical features, type of malignancy, family history, and outcome. The patients were treated as per the discretion of the treating physician and all investigations advised by the treating physician were noted in the case record form including complete blood count, blood markers, bone marrow examination, liver and kidney function tests, etc. No investigations were carried out as a part of the study. For the analysis of the data, the analytical software Statistical Package for the Social Sciences (SPSS) SPSS23 was used. Results of demographic and biochemical characteristics were expressed as range, mean, and median.
RESULTS
During the study duration of 18 months, 80 patients fulfilling the inclusion criteria were enrolled in the study. The majority of cases were from the age group 36–50 years, i.e., 32 in number (40%) followed by 51–65 years (25%). Eleven (12.8%) cases were from the age group 21–35 years. A greater number of male patients were encountered than female ones (67.5% vs 32.5%). For both sexes, most cases belonged to the age group 36–65 years [Figure 1].

- Distribution of study participants according to their demographic characteristics—age and sex.
Most of the patients were unskilled laborers followed by semi-skilled workers. Pesticide exposure was the commonest etiological factor reported in 16 (20%) patients, followed by exposure to silica, which was reported in nine (11.25%) patients [Figure 2].
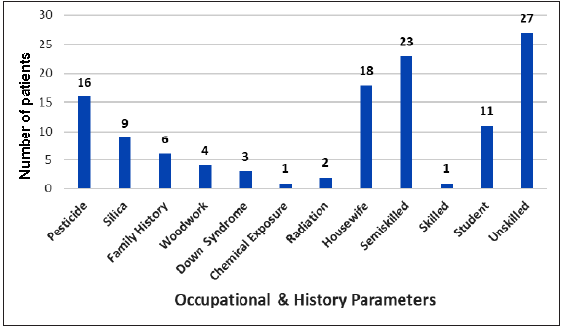
- Probable causative factors among the study participants.
The most common symptoms reported were fatigue (93.75%) and fever (93.5%) followed by weight loss (35%), anorexia (33.75%) and abdominal pain (23.75%) while among the clinical signs, pallor (92.50%) was most commonly observed followed by lymphadenopathy (21.30%), sternal tenderness (12.50%) and oedema (11.30%) [Table 1].
| Sr. No. | Clinical features (Symptoms) | Frequency | Percentage |
|---|---|---|---|
| 1 | Fatigue | 75 | 93.75 |
| 2 | Fever | 75 | 93.75 |
| 3 | Weight Loss | 28 | 35.00 |
| 4 | Anorexia | 27 | 33.75 |
| 5 | Abdominal Pain | 19 | 23.75 |
| 6 | Myalgia | 16 | 20.00 |
| 7 | Dyspnoea | 16 | 20.00 |
| 8 | Cough | 12 | 15.00 |
| 9 | Headache | 06 | 07.50 |
| 10 | Rash | 06 | 07.50 |
| 11 | Arthralgia | 05 | 06.25 |
| 12 | Hematemesis | 04 | 05.00 |
| 13 | Malena | 05 | 06.25 |
| Sr. No. | Clinical features (Signs) | Frequency | Percentage |
| 1 | Pallor | 74 | 92.50 |
| 2 | Lymphadenopathy | 17 | 21.30 |
| 3 | Sternal Tenderness | 10 | 12.50 |
| 4 | Oedema | 09 | 11.30 |
| 5 | Pathological Fracture | 06 | 07.50 |
| 6 | Purpura | 02 | 02.50 |
| 7 | Gum Bleeding | 01 | 01.30 |
Out of the 80 cases enrolled in the study, 42 (52.5%) patients were of AML, followed by 15 (18.8%) patients of Non-Hodgkin’s lymphoma (NHL) and 8 (10%) patients had multiple myeloma as seen in Figure 3. The association between the type of malignancy and age group, sex, and family history of malignancy was found to be statistically significant [Table 2].
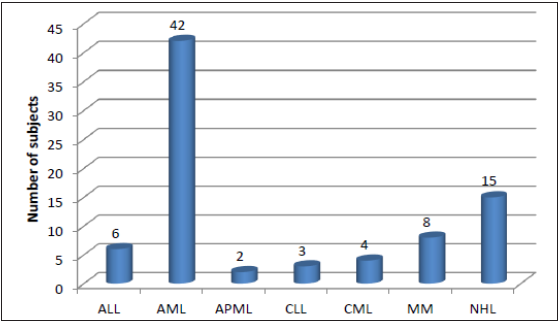
- Distribution of study subjects according to the type of malignancy. ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; APML: Acute Promyelocytic Leukemia; CLL: Chronic Lymphocytic Leukemia; CML: Chronic Myeloid Leukemia; MM: Multiple Myeloma; NHL: Non-Hodgkin Lymphoma.
| Type of malignancy | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| ALL | AML | APML | CLL | CML | MM | NHL | ||
| Total | 6 | 42 | 2 | 3 | 4 | 8 | 15 | 80 |
| Age (years) | ||||||||
| ≤20 | 4 | 3 | 2 | 0 | 0 | 0 | 0 | 9 |
| 21–35 | 0 | 9 | 0 | 1 | 0 | 0 | 1 | 11 |
| 36–50 | 1 | 21 | 0 | 0 | 1 | 3 | 6 | 32 |
| 51–65 | 1 | 8 | 0 | 1 | 3 | 3 | 4 | 20 |
| >65 | 0 | 1 | 0 | 1 | 0 | 2 | 4 | 8 |
| Sex | ||||||||
| Male | 4 | 20 | 2 | 3 | 4 | 7 | 14 | 54 |
| Female | 2 | 22 | 0 | 0 | 0 | 1 | 1 | 26 |
| Family history of Malignancy | ||||||||
| Yes | 2 | 0 | 0 | 3 | 0 | 0 | 1 | 6 |
| No | 4 | 42 | 2 | 0 | 4 | 8 | 14 | 74 |
ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; APML: Acute Promyelocytic Leukemia; CLL: Chronic Lymphocytic Leukemia; CML: Chronic Myeloid Leukemia; MM: Multiple Myeloma; NHL: Non-Hodgkin Lymphoma.
All the patients were referred to a higher center (Tata Memorial Hospital) on diagnosis. However, few succumbed to the illness while they were admitted to the tertiary healthcare center (King Edward Memorial Hospital). Table 3 depicts the association between the outcome of the case and age group, and sex and family history of malignancy of the patient.
| Age (years) | Outcome | Total (n = 80) | |
|---|---|---|---|
| Referred | Death | ||
| ≤20 | 8 | 1 | 9 |
| 21–35 | 10 | 1 | 11 |
| 36–50 | 24 | 8 | 32 |
| 51–65 | 18 | 2 | 20 |
| >65 | 6 | 2 | 8 |
| Gender | |||
| Male | 40 | 14 | 54 |
| Female | 26 | 0 | 26 |
| Total | 66 | 14 | 80 |
| Family History | |||
| Yes | 5 | 1 | 6 |
| No | 61 | 13 | 74 |
| Total | 66 | 14 | 80 |
n: number of patients.
DISCUSSION
A total of 80 patients with hematological malignancies were studied in this study. The patients in the age group of 36–50 years were most common followed by the 51–65 years’ group. It concurs with the findings of Nwannadi IA et al.[3] who reported that hematological malignancies were most common in the >15 age group. Shafaq Maqsood[4] observed a median age of 36 years. India has a relatively young population and the population over age of 65 years is still <5%, which is reflected in the demographics of diseases observed in the country.[14]
This parallels the global trend in hematological malignancies.[15] The findings of Nwannadi IA et al.,[3] Babatunde et al.,[5] Onwuasigwe et al.,[6] Al Lamki Z et al.,[7] Omoti CE et al.,[8] Sayers GM et al.,[9] Radha Rathee et al.,[10] Rodriguez-Abreu.,[11] Shafaq Maqsood.,[4] Mava Yakubu et al.[12] all found a male preponderance in the incidence of hematological malignancies.
In the present study, 33.75% (27/80) patients were unskilled, while 28.75% (23/80) were semiskilled by occupation. Paolo P et al.[13] demonstrated that populations at a significant risk for hematological malignancies were farmers and industrial workers. In this study, 20% cases were exposed to pesticides, 11.25% cases were to silica and 7.50% cases were to woodwork, while six (7.50%) were having a family history of malignancy and three (3.75%) were having Downs syndrome. As reported by Alvanja MC et al.,[16] Merhi M et al.[17] and Levine EG et al.[18] exposure to asbestos, aromatic hydrocarbons, fertilizers, mineral oils, pesticides and radiation have been reported to be associated with a significant risk for malignant diseases. Maria Kokouva et al.[19] observed that 28% lympho-hematoid cancers are associated with pesticide exposure. India is a developing country with a part of our population being illiterate and engaged in farming and labor. Low awareness about precautions needed while handling pesticides and chemicals, along with less use of protective equipment because of cultural and economic reasons, contributes to pesticide and chemical exposure.
In this study, the common presenting symptoms were fatigue (93.75%), fever (93.5%), weight loss (35%), and anorexia (33.75%). Abdominal pain (23.75%), myalgia (20.00%) and dyspnoea (20.00%) were also present. The most common clinical sign in this study was pallor (92.50%) followed by fever (48.80%), lymphadenopathy (21.30%), sternal tenderness (12.50%) and oedema (11.30%). AS Islam et al. reported anemia as the commonest sign[20] while Radha Rathee et al.[10] observed that the commonest presenting symptoms for leukemia were low-grade fever, progressive pallor, weakness and body ache (70% cases) while the most frequently observed sign was pallor. Proper training of clinicians and maintaining an index of suspicion regarding the possibility of malignancy will ensure that clinical signs will be picked up early, resulting in early intervention and better outcomes.
In this study, AML was found to be the most common malignancy (52.5%), similar to the findings of Kumar et al.,[21] followed by NHL (18.8%), Multiple Myeloma (MM) 8(10%), ALL (7.5%), CLL (3.8%), Acute Promyelocytic Leukemia (APML) (2.5%). Other Indian studies had reported a greater incidence of Chronic Myeloid Leukemia (CML), analogous to studies by Radha Rahee et al.[10] and Samrat S et al.[22] This difference could be explained by the presence of geographical variations in incidence and the fact that King Edward Memorial Hospital, where this study was conducted, is a tertiary care center with a large number of referrals. Nwannadi IA et al.[3] observed that 48.1% of all hematological malignancies encountered in West Africa were lymphomas. Of this, NHL was also observed in the studies by Williams CKO et al.,[23] Hartge P et al.[24] and Anderson RE et al.[25] Tenge et al.[26] reported that the most common hematological malignancy was lymphomas. Damulak Obadiah Dapus et al.[27] in their study on the pattern of leukemia observed that the frequency of individual cases was CLL (32.5%), CML (31.6%), AML (19.0%), and ALL (16.9%). Babatunde et al.[5] in their 10-year review study observed that the recorded distribution of the various hematological malignancies were ALL 18 (4.9%), AML 18 (4.9%), CLL 20 (5.4%), CML 42 (11.4%), hairy cell leukemia 2 (0.5%), NHL 104 (28.1%), Hodgkin’s disease 42 (11.4%), Burkitt’s lymphoma 102 (27.5%), multiple myeloma 20 (5.4%) and plasma cell leukemia 2 (0.5%).
In this study, the prevalence of AML was more in the 36–50 years age group, the prevalence of ALL was more in the <20 years age group and the prevalence of non-Hodgkin’s lymphoma was more in the 36–65 years age group, which was similar to the findings of Dong Y. et al. globally in 2020.[28] Nwannadi IA et al.[3] observed that the prevalence of ALL was the highest among those aged 0–20 years.
The association between family history and the type of malignancy was statistically significant with familial association in chronic lymphocytic leukemia (3/80), acute lymphocytic leukemia (2/80), and in one patient of NHL. Shirley V Hodgson et al.[29] reported that an increased risk to relatives was marked in chronic lymphocytic leukemia, less so in acute lymphocytic leukemia, and absent in chronic myeloid leukemia. Martha S Linet et al.[30] had observed that a family history of CLL correlated with increased risks of leukemia as well as other haemato-lymphoproliferative.
In this study, mortality was 17.5% (14) and 66 (82.5%) were referred to the Tata Memorial Hospital for further treatment. The association between age group and family history with the outcome was not statistically not statistically significant. Males had a worse outcome which was statistically significant—mortality rate in male patients is 25.9% (14/54) and in females it is 0% (0/26). In adult malignancies, males have reported a worse prognosis and significantly higher mortality rate according to a study conducted by Molife et al.[31]
LIMITATIONS
The shorter duration of the study resulted in a smaller sample size. Subsequent studies must be undertaken for a longer duration to validate these findings.
CONCLUSION
The common age group to encounter hematological malignancy in patients is 36–50 years, being more prevalent among males. History of exposure to risk factors such as pesticides is commonly seen. Age and family history do not correlate with the outcome. Females tend to have a better outcome than males.
Ethical approval
Approval from the Institutional Ethics Committee was obtained before the commencement of the study, approval reference number IEC/209/2015.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCE
- Outcomes of Critically Ill Patients with Hematologic Malignancies: Prospective Multicenter Data from France and Belgium. J Clin Oncol. 2013;31:2810-8.
- [CrossRef] [PubMed] [Google Scholar]
- Global, Regional, and National Burdens of Leukaemia from 1990 to 2017: A Systematic Analysis of the Global Burden of Disease 2017 Study. Aging (Albany NY). 2021;13:10468-89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The Epidemiology of Haematological Malignancies at the University of Benin Teaching Hospital: A Ten-Year Retrospective Study. Int J Epidemiol. 2010;9:1-6.
- [Google Scholar]
- Characteristics and Outcomes of Patients with Hematological Malignancies Admitted for Intensive Care - a Single Centre Experience. Asian Pac J Cancer Prev. ;18:1833-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pattern of Haematological Malignancies in Ilorin, Nigeria: A Ten-Year Review. Internet J Haemat. 2008;5:1-7.
- [Google Scholar]
- Spectrum of Paediatric Malignancies in Eastern Nigeria (1989–1998) West Afr J Med. 2002;21:31-33.
- [PubMed] [Google Scholar]
- Plasma Cell Myeloma in a Tertiary Centre in the Niger Delta Region of Nigeria Clinico-immunologic Analysis. Pak J of Med Sci. 2006;23:27-32.
- [Google Scholar]
- Epidemiology of Acute Leukaemia in the Cape Province of South Africa. Leuk Res. 1992;16:961-6.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of Acute and Chronic forms of Leukemia in Haryana. Int J of Pharmacy and Pharmaceutical Sciences. 2014;6:323-5.
- [Google Scholar]
- Prevalence and Clinical Manifestation of Lymphomas in North Eastern Nigeria. Indian J Cancer. 2015;52:551-5.
- [CrossRef] [PubMed] [Google Scholar]
- The Growing Burden of Cancer in India: Epidemiology and Social Context. Lancet Oncol. 2014;15:e205-12.
- [CrossRef] [PubMed] [Google Scholar]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Occupational Pesticide Exposures and Cancer Risk: A Review. J Toxicol Environ Health B Crit Rev 2012
- [Google Scholar]
- Occupational Exposure to Pesticides and Risk of Hematopoietic Cancers: Meta-Analysis of Case-Control Studies. Cancer Causes Control. 2007;18:1209-26.
- [CrossRef] [PubMed] [Google Scholar]
- Leukaemia and Myelodysplastic Syndrome Secondary to Drugs, Radiations and Environmental Exposure. Semin Oncol. 1992;19:47-51.
- [Google Scholar]
- Pesticide Exposure and Lymphohaematopoietic Cancers: A Case-Control Study in an Agricultural Region (Larissa, Thessaly, Greece) BMC Public Health. 2011;11:5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinicopathological Findings of Haematological Malignancies in Hospital Admitted Patients. Mymensingh Med J. 2021;30:28-34.
- [PubMed] [Google Scholar]
- Hematological Malignancies in Relation with Abo Blood Group at a Teaching Hospital, Varanasi, India. J Family Med Prim Care. 2020;9:2309-12.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of Chronic Myeloid Leukemia with Generic Imatinib in Patients from Northeastern Part of India. Int J Res Pharm Sci. 2019;10:3107-13.
- [Google Scholar]
- Estimation of Incidence of Human Leukaemia Subtypes in an Urban African Population. Onco. 1983;40:381-6.
- [Google Scholar]
- Geographical Aspect of Malignant Lymphoma and Multiple Myeloma. Selected Comparisons Involving Japan, England and the United States. Am J Path. 1970;61:85-97.
- [PubMed] [PubMed Central] [Google Scholar]
- The Pattern of Leukaemias Among Adults in Jos, North Central Nigeria, Remedy Publications LLC. 2017. ;1 Article 1001, 1–6
- [Google Scholar]
- Leukemia Incidence Trends at the Global, Regional, and National Level between 1990 and 2017. Exp Hematol Oncol. 2020;9:14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Practical Guide to Human Cancer Genetics. London: Springer; 2014.
- Familial Cancer History and Chronic Lymphocytic Leukemia: A Case-Control Study. Am J Epidemiol. 1989;130:655-64.
- [CrossRef] [PubMed] [Google Scholar]
- Gender and Survival in Malignant Tumours. Cancer Treat Rev. 2001;27:201-9.
- [CrossRef] [PubMed] [Google Scholar]







