Translate this page into:
ACARDIAC – ACEPHALUS TWIN – A RARE CASE REPORT AND REVIEW OF LITERATURE
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
How to cite this article – Mital M, Garg T, Gupta P et. al. Acardiac – Acephalus twin – A rare case report and review of literature, IJRSMS, 2015;01(1): 8 - 11
Abstract
Acardiac twin pregnancy is a rare complication of monochorionic twinning caused by aterioarterial and venovenous placental anastomosis, leading to circulatory predominance of one twin (1). The donor “pump’ twin provides circulation for itself as well as and for the recipient acardiac twin. The acardiac twin is usually grossly abnormal with severe reduction anamolies of the upper part of the body. We present a case of acephalus acardiac anomaly diagnosed by ultrasound, with subsequent post delivery followup.
Keywords
Acardiac twin
Ultrasound
Acephalus
INTRODUCTION
Acardia is a rare anomaly that is unique to monochorionic twins. This developmental anomaly consists of a wide spectrum of malformations usually including absence of many organs in thorax and abdomen, typically with a lack of functional heart (hence the name acardia). It has been proposed that the anomaly develops as a result of extensive artery-to-artery and vein-to-vein anastomosis in the twin placenta leading to perfusion of the abnormal twin (perfused acardiac twin) from the co twin (pump twin). It is now also known as Twin reversed arterial perfusion syndrome (TRAP). Acardiac acephalus is the most common type of acardiac twin. The major perinatal problems associated with TRAP are congestive cardiac failure, hydrops of the normal twin, and preterm delivery.
CASE PRESENTATION
A 22-year-old primigravida was referred to us for a routine antenatal ultrasound. The patient had undergone a previous ultrasound about two days back which had been reported as a normal singleton live foetus, with an adjacent soft tissue mass, probably as dead twin. At our institution, ultrasound was repeated and demonstrated a twin pregnancy without any intertwin membrane with the following additional findings.
TWIN A was found to be in vertex presentation with a normal foetal anatomy consistent with 23 weeks gestation. There was no foetal ascites or pleural effusion.
TWIN B [reported as a soft tissue mass by the previous sonographer] had grossly abnormal anatomy. The head and neck were absent. There was no definition of foetal skull above the thorax with the spine ending abruptly (Figure 1).
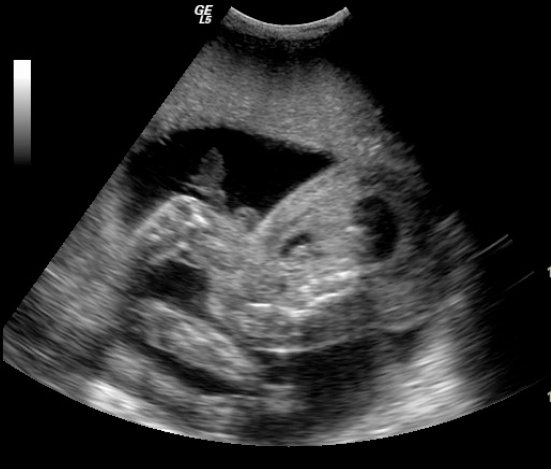
- Long axis of Twin B showing absent head and neck with the spine ending abruptly
The upper limbs were small and ill developed, while the lower limbs were well-developed and showed movements. One of the lower limbs showed clubfoot. NO IDENTIFIABLE CARDIAC STRUCTURES WERE PRESENT. Multiple cystic cavities were seen in the thorax. There was a marked multiloculated cystic soft tissue swelling in the region of thorax and abdomen. (Figure 2-3)

- Transverse view of Twin B showing multiple cystic cavities in thorax and marked multiloculated cystic soft tissue swelling in the region of thorax and abdomen. No identifiable heart.

- Transverse view of Twin B showing multiple cystic cavities in thorax and marked multiloculated cystic soft tissue swelling in the region of thorax and abdomen. No identifiable heart.
The patient was explained about the prognosis and risk for preterm labor. One week later she went into spontaneous labour and delivered stillbirth twins (Figure 4-5) show the products of delivery, which confirm the diagnosis of acardiac twin pregnancy. Additionally the cord of acardiac twin was much thinner than the other. The patient, however, refused a subsequent autopsy.

- Products of delivery showing Twin A with normal anatomy and Twin B with absent head and neck, small ill developed upper limbs, well developed lower limbs and extensive soft tissue swelling
There was moderate associated polyhydroamnios. The Doppler studies in umbilical arteries failed to demonstrate any significant abnormality in normal fetus, probably due to early gestation. The placenta was single. The ultrasound findings were consistent with the diagnosis of Acardiac twin pregnancy.
DISCUSSION
Acardia is a rare complication of monochorionic twinning and is reported in 1 of 35,000 births (1).
It is also known as Twin Arterial Perfusion Sequence [TRAP]. It is caused by arterio-arterial and venovenous placental anastomosis causing a physically normal foetus [Donor Twin] to circulate blood for both itself and a severely malformed foetus [Acardiac twin].
While the antenatal diagnosis of TRAP has been reported by several authors (2-6), its pathogenesis remains controversial. Evidence suggests that intrauterine growth is achieved by perfusion from the normal twin via a large vessel anastomosis in the placenta. Perfusion of the anamolous twin occurs by reversal of flow through the umbilical arteries (7). Because the acardiac twin's body is only perfused by the pump twins arterial blood, the acardiac twin has reduced blood oxygen saturation levels (8). As the acardiac twin receives poorly oxygenated blood through the umbilical arteries, the structures supplied by the iliac artery and distal abdominal aorta are well perfused, while the upper half of the body is not, so the acardiac twin can have anencephaly, small head, absent/hypoplastic upper torso and limbs, absent/anamolous two chambered heart, multiloculated dorsal cystic hygroma (9,10,11)
Four traditional categories of Acardiac twins have been described (12), based on the degree of cephalic and truncal maldevelopment, namely Acardius Acephalus, Acardius Anceps, Acardius Acormus and Acardius Amorphus.
Acardius acephalus is the most common type (as in our case). These twins have no head, they have legs. On autopsy they are found to lack chest and upper abdominal organs. Acardius anceps is the most developed form of this anamoly. This form has arms, legs, and a partially developed head and/or neural tissue. Acardius amorphus is the most seriously maldeveloped type which retains virtually no cephalic/truncal organization at all (2).
Early ultrasound detection of the TRAP sequence may be difficult. The acardiac twin may be mistaken for a foetal demise because no cardiac activity is present. Repeat studies document the acardiac twin. The acardiac twin typically has some recognizable foetal features such as limbs or bones but there is no cardiac activity and major anomalies are a rule. Careful ultrasound examination of the placental surface of the acardiac twin can identify vascular connection to the donor twin through arterial-to-arterial anastomosis (13).
MANAGEMENT
Management of TRAP syndrome should include serial ultrasounds to assess the growth rate and cardiovascular status of the normal twin. The size of the recipient twin relative to the acardiac twin, cardiac dysfunction / hydrops of the donor twin and Doppler ultrasound of the communicating vessels predict the outcome for the donor twin and help to direct treatment during pregnancy (14,15)
These data may be useful in management of these pregnancies and in deciding the need for prenatal treatment As the morbidity and mortality for the pump twin depends on the degree of perfusion of the acardiac twin, it seems evident that any decrease or interruption of the blood flow to this tissue will have a beneficial therapeutic effect. A variety of techniques have been used to achieve this with varying success. These techniques include fetoscopic laser ablation of the vessels in the cord to the acardiac twin [16], fetoscopic cord ligation (17), intravascular alcohol injection to sclerose the vessel (18), hysterectomy to remove the acardiac twin (19), bipolar cord coagulation (20), and RFA (21). The therapies most commonly used currently are laser, bipolar coagulation and RFA, with survival rates of (80-92%) (22-23)
Conflict of Interest
NIL
REFERENCES
- Acardiac twin with preserved brain. Foetal Diag Ther. 2001;16:231-233.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal sonographic diagnosis and management of a twin pregnancy with placenta previa and hemicardia. Am J Perinat. 1987;4:313-6.
- [CrossRef] [PubMed] [Google Scholar]
- Europ J Obstet Gynecol Reprod Biol. 1985;19:317-25.
- [CrossRef] [PubMed]
- Prenatal sonographic diagnosis Doppler velocimetric umbilical cord studies and subsequent management of an acardiac twin pregnancy. Obstet Gynecol. 1989;74:472-75.
- [Google Scholar]
- Antenatal diagnosis of acephalus acardia : a proposed management scheme. Am J Obstet Gynecol. 1983;143:857-59.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal identification of twin reversed arterial perfusion syndrome in the first trimester. Am J Obstet Gynecol. 1989;160:1194-96.
- [CrossRef] [PubMed] [Google Scholar]
- Modeling acardiac twin pregnancies. Ann N Y Acad Sci. 2007;1101:235-49.
- [CrossRef] [PubMed] [Google Scholar]
- Twin gestations. In: Diagnostic ultrasound of fetal anamolies Text and Atlas. Chicago Yr Book of medical publishers; 1990. p. :592-622.
- [Google Scholar]
- Monochorionic twinning: Sonographic assessment. AJR. 1990;154:459-69.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonic evaluation in multiple gestation.Usg in Obs and Gynae. (3rd edition). Philadelphia: W.B. Saunders; 1994. p. :102-28.
- [Google Scholar]
- Doppler demonstration of reversed umbilical blood flow in an acardiac twin. J Clin Ultrasound. 1989;17:291-95.
- [CrossRef] [PubMed] [Google Scholar]
- Perinatal outcome of 49 pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907-12.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of Doppler velocimetry in predicting outcome in Twin reversed arterial perfusion sequence. Am J Obstet Gynecol. 2001;185:135-39.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic laser coagulation of umbilical cord vessels in twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 1994;4:396.
- [CrossRef] [PubMed] [Google Scholar]
- Foetal endoscopic surgery in a case of twin pregnancy complicated by reversed arterial perfusion sequence (TRAP sequence) Rev Chil Obstet Ginecol. 1995;60:112.
- [Google Scholar]
- Ablation of acardiac twin by alcohol injection into the intra-abdominal umbilical artery. Obstet Gynecol. 1995;86:680.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of acardiac acephaluc twin gestations by hysterotomy and selective delivery. Obstet Gynecol. 1992;79:601.
- [Google Scholar]
- Bipolar Coagulation of the umbilical cord in complicated monochronic twin pregnancy. Am J Obstet Gynecol. 2000;182:340.
- [CrossRef] [PubMed] [Google Scholar]
- Selective reduction of acardiac twin by radio-frequency ablation. Am J Obstet Gynecol. 2002;182:635.
- [CrossRef] [PubMed] [Google Scholar]
- Twin reversed arterial perfusion: Fetoscopic laser coagulation of placental anastomosis of the umbilical cord. Ultrasound Obstet Gynecol. 2006;28:688.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of radiofrequency ablation for twin reversed arterial perfusion sequence. Am J Obstetics Gynecol. 2007;196:459.
- [CrossRef] [PubMed] [Google Scholar]







