Translate this page into:
Assessment of Laparoscopic Posterior Mesh Rectopexy for Complete Rectal Prolapse: A Case Series with Review of Literature
Address for correspondence Vishal Chawda, MS, Department of General and Laparoscopic Surgery, Dr L H Hiranandani Hospital, Powai, Mumbai 400076, India (e-mail: drvishalchawda@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Trans-abdominal rectopexy for complete rectal prolapse (CRP) reportedly yields more definitive results as compared with trans-perineal surgery. In the era of minimal access surgery, minimally invasive laparoscopic rectopexy has become a popular treatment option for patients with rectal prolapse (RP). Herein, we describe our preferred surgical procedure for the correction of RP and evaluate its results. We further aim to perform a comparative assessment between perioperative outcomes achieved with open and laparoscopic applications of this technique.
Materials and methods
This was a retrospective cross-sectional observational study conducted at a tertiary health care center in Maharashtra, India. We studied cases of RP who underwent laparoscopic posterior mesh rectopexy during the past 15 years (2005–2021), in our institution, operated upon by a single surgeon.
Results
Of the total 14 patients, 12 were managed with laparoscopic posterior mesh rectopexy. The remaining two underwent laparoscopic suture rectopexy. The mean operative time was 120 minutes. Constipation improved among 28.57%, remained the same among 21.42%, and worsened among 35.71% patients. No intra-operative blood transfusion was required. Mean length of hospital stay was 4 days. There were no recurrences over a mean follow-up period of 94 months, i.e., 7.83 years (range 7 – 197 months).
Conclusions
Laparoscopic posterior rectopexy can be safely performed in older patients to achieve early postoperative ambulation and significantly shorten the hospital stay. It may, therefore, be considered an effective treatment for CRP and urinary dysfunction. However, the incidence of de-novo constipation and worsening of pre-existing constipation is significantly high.
Keywords
rectal prolapse
fecal incontinence
laparoscopic posterior mesh rectopexy
Introduction
Complete rectal prolapse (CRP) is defined as the circumferential and full-thickness protrusion of the rectum out of the anal verge. Surgical techniques described for CRP include resection, rectopexy, and combined resection rectopexy.1 Also described are mucosal stripping with plication of rectum, Thiersch's stitch, etc. Recently, minimally invasive surgery for rectal prolapse (RP) repairs has gained wide acceptance because of advantages like relatively easier and magnified access to the pelvic recess and floor, decreased operative pain, faster recovery, and early discharge.2 Different laparoscopic techniques described are sutureless rectopexy, suture rectopexy, procto-sigmoidectomy, and mesh rectopexy.
A vast number of surgical procedures are described for RP, in the literature. The ultimate goal is to treat first and then prevent recurrence and to restore normal defecation function. However, the utopian achievement of all these three goals is beyond the reach of any single procedure. Perhaps, it is for this one reason that so many surgical procedures exist, in the first place.
Material and Methods
This was a retrospective cross-sectional observational study conducted at a tertiary health care center in Maharashtra, India. We evaluated patients of CRP and our experience with laparoscopic posterior mesh rectopexy, during the past 15 years (2005 to 2021). All the patients were operated upon by a single surgeon.
Patients were diagnosed based on clinical examination findings. Demographic data, medical history, and surgical and follow-up details of the patients were recorded and are summarized (►Table 1).
| Characteristics of patients | Numbers |
|---|---|
| Total number of patients | 14 patients |
| Mean age | 48.42 y |
| Sex ratio (M:F) | 1.8:1 |
| Clinical presentation–associated symptoms in addition to SCOPR | |
| Constipation | 13(93%) |
| Painful evacuation | 4 (28.57%) |
| Rectal bleeding | 2 (14.28%) |
| Mean operating time | 120 min |
| Laparoscopic posterior mesh rectopexy | 12 patients (86%) |
| Laparoscopic suturerectopexy | Two patients (14%) |
| Median hospital stay | 5 d |
Abbreviation: SCOPR, something coming out per rectum.
Patients were first evaluated 2 weeks postoperatively and were then followed-up for the evaluation of postoperative sequelae and complications including constipation and recurrence, at 1, 3, 6, and 12 months in the postoperative period. The pre- and post-operative constipation was objectively measured using the Wexner score (►Fig. 4 and ►Table 2). Those patients who failed to physically follow-up beyond the immediate postoperative follow-up visit were interviewed telephonically. At the time of writing this paper, a telephonic interview was conducted with all the patients.
| Preop. avg WS | 13 patients–12 | One patient–0 | |||
|---|---|---|---|---|---|
| Postop. avg WS | |||||
| Post op. day | Constipation improved | New onset constipation | Constipation remained the same | Worsening of pre-existing constipation | |
| 15 d | 10 (two patients) | 0 | 12 (six patients) | 14 (five patients) | |
| 1 mo | 9 (three patients) | 0 | 12 (five patients) | 16 (five patients) | |
| 3 mo | 7 (four patients) | 9 (one patient) | 12 (five patients) | 17 (four patients) | |
| 6 mo | 5 (four patients) | 8 (one patient) | 12 (five patients) | 16 (four patients) | |
| 12 mo | 6 (two patients) | 9 (one patient) | 12 (four patients) | 20 (seven patients) | |
Abbreviations: avg., average; Postop., postoperative; Preop., preoperative; WS, Wexner score.
All patients received a preoperative bowel preparation before surgery, i.e., Peglec 1 packet dissolved in 2 L of water to be consumed over 2 hours on the previous day between 2 and 4 pm. As per the hospital antibiotic policy, Ceftriaxone 1 g and Metrogyl 500 mg were administered intravenously, just prior to incision. A urinary catheter was placed after induction of general anesthesia. The procedures were performed with the patient placed in supine position and strapped firmly to the table, so as to allow the steep Trendelenburg position. The operation was performed using four trocars: a 10-mm trocar at the umbilicus, a 5-mm trocar in the left upper quadrant, a 12-mm trocar in the right iliac fossa, and a 10-mm trocar in the right upper quadrant. The surgeon stood to the right side of the patient, with the camera assistant on the upper right side of the patient and the second assistant on the left side.
In female patients, the uterus was fixed to the anterior abdominal wall using 2-0 nylon. ►Fig. 1B shows the initial incision of the posterior parietal peritoneum at the level of the sacral promontory, so as to enter the extra-peritoneal space. An inverted J-shaped peritoneal opening was created along the right posterio-lateral to the anterior side of the rectum from the sacral promontory to the cul-de-sac (►Fig. 1D). Posterior dissection was performed for rectal mobilization along the plane of the fascia propria of the rectum through the retro-rectal avascular space from the sacral promontory to the coccyx, toward the pelvic floor (►Figs. 1C and 2A). The lateral ligament was divided to enable better mobilization. The anterior dissection was performed at the upper third portion of rectum. A similar incision was then made on the left side and deepened so as to enter the already developed pre-sacral space.

- Examination findings and operative pics. (A) Complete full thickness rectal prolapse. (B) Initial incision of posterior parietal peritoneum with entry into extraperitoneal space. (C) Caudad progression of incision toward the pelvic floor in the retrorectal “holy” plane (red asterisk). (D) Caudad progression of right lateral posterior parietal incision.

- Operative pics. (A) Completed dissection in the retro-rectal, pre-sacral plane down to the pelvic floor (red asterisks). (B and C) Optimum placement of polypropylene mesh in the pre-sacral space and tacker fixation of mesh to the endopelvic fascia. (D) Right margin of the mesh being suture fixed to the rectal musculosa, while holding the rectum in proximal traction.
Polypropylene mesh (PROLENE, Ethicon Inc. Somerville, NJ, United States) of size 15 × 7.6 cm was rolled and introduced into the abdominal cavity through the 12-mm trocar. The rectum was lifted by the assistant, and the mesh was fixed to the periosteum of the sacral promontory and lower down to the pre-sacral fascia using a tacker (PROTACK, Covidien Inc.) at three places (►Fig. 2B and C). The rectum was then held under slight proximal traction and its musculosa was sutured to the two lateral borders of the mesh, using 2-0 polypropylene with three stitches on either side (►Fig. 2D). In the end, the mesh was extra-peritonealized by suture closure of the peritoneal defects on the two sides using 2-0 Vicryl with continuous interlocking sutures (►Fig. 3A – D).

- Operative pics. (A, B, and C) Extraperitonization of mesh by suture closure of the created right sided peritoneal defect. (D) The same being done for the created left sided peritoneal defect.

- Wexner score.
Statistical Analysis
The data were collected with the help of standard, pre-validated, semi-structured case record proforma, from the hospital records and through a telephonic questionnaire. They were then entered using MS excel software. The data were analyzed using Statistical Package for the Social Sciences version 22 software. They were represented with the help of tables and charts for frequency analysis.
Results
In the present study, we assessed the demographic features of all the 14 study subjects who presented with RP and were operated with laparoscopic posterior mesh rectopexy and suture rectopexy during the past 15 years (2005 – 2021). We observed that the mean age of the subjects was 48.42 years with a standard deviation of 20.7 years while median age of presentation was 50 years. There was a male preponderance observed in the current study, which is against a well-established female preponderance in the disease. The observed M:F ratio was 1.8:1.
In the current study, we assessed the clinical presentation of RP among the study subjects. We observed that along with the obvious chief complaint of something coming out per rectum, among associated complaints, the commonest was constipation (n = 13, 93%), followed by painful evacuation (n = 4, 28.57%), and rectal bleeding (n = 2, 14.28). None of the patients in this series had incontinence as an associated symptom. The mean duration of pre-operative symptoms among the study subjects was found to be 18.5 weeks. Majority of the patients (n = 12, 85.71%) were managed with laparoscopic posterior mesh rectopexy, while two patients (14.29%) were managed with laparoscopic suture rectopexy. The mean operative time was 120 minutes. No intra-operative blood transfusion was required. Median length of hospital stay was 5 days (range, 3 – 7 days). There was no peri-operative mortality. No mesh-related complications such as mesh infection, erosion, etc., were observed. There were no other complications noted, such as injury to ureter/s or major blood vessels. There were no conversions to open surgery. There were no recurrences observed in the study population.
At median follow-up of 15 months, constipation improved among 2 (15%), remained the same among 4(31%), and worsened among 7 (54%) patients. In both the cases of suture rectopexy, it improved with time. The follow-up findings are summarized in ►Table 3.
| Follow-up period | Constipation improved | New onset constipation | Constipation remained same | Worsening of pre-existing constipation | Recurrence |
|---|---|---|---|---|---|
| 15 d | 2 (15%) | 0 (0%) | 6 (46%) | 5 (38%) | 0 (0%) |
| 1 mo | 3 (23%) | 0 (0%) | 5 (38%) | 5 (38%) | 0 (0%) |
| 3 mo | 4 (31%) | 1 (100%) | 5 (38%) | 4 (31%) | 0 (0%) |
| 6 mo | 4 (31%) | 1 (100%) | 5 (38%) | 4 (31%) | 0 (0%) |
| 12 mo | 2 (15%) | 1 (100%) | 4 (31%) | 7 (54%) | 0 (0%) |
Discussion
RP can be internal (also called recto-rectal intussusception: Grades 1 – 4 of Oxford Rectal Prolapse Grading System–ORPGS) or external (Grade 5 of ORPGS) (►Fig. 5). Generally, patients of external RP (►Fig. 1A) have pain, bleeding, constipation, and/or incontinence as associated symptoms in addition to the main obvious symptom of something coming out per rectum. Patients with internal RP have obstructed defecation or fecal incontinence. External RP requires a surgical correction. However, internal RP may be treated conservatively in the beginning (lifestyle modifications, appropriate diet, pelvic physiotherapy, bio-feedback, psychological-psychiatric counseling where applicable, etc.) and then eventually repaired surgically, if there is no satisfactory symptomatic response.3 The preferred surgery as of present day, for internal RP is laparoscopic ventral mesh repair (D'Hoore et al), if medically fit for general anesthesia and no local contraindications to abdominal procedure such as endometriosis, history of pelvic radiation, major pelvic surgery in the past, severe pelvic inflammatory disease, etc. If abdominal surgery is contraindicated due to any of the above reasons, stapled transanal resection of rectum (STARR) and Delorme operation are surgical options.3 Genital prolapse or pelvic organ prolapse can be associated with RP in up to 30% patients. Hence, a vaginal examination is essential in female patients with RP.4 In general, ano-rectal function tests are only indicated pre-operatively for patients with obstructed defecation/fecal incontinence where internal RP is suspected.3 Among specific preoperative investigations, magnetic resonance imaging defecography is advised for patients with obstructed defecation syndrome, suspected to have internal RP (ORPGS grades I-IV).4 Ano-rectal manometry should be done for patients with reducible external RP with fecal incontinence and also for patients with obstructed defecation and/or fecal incontinence with suspected internal RP.4 Neurophysiological testing is required if RP is associated with central and peripheral neurological diseases.4 By and large, for patients with CRP/procidentia (all patients of our series), no specific functional tests are required, except for a small subset of patients with reducible external RP with incontinence, described above.
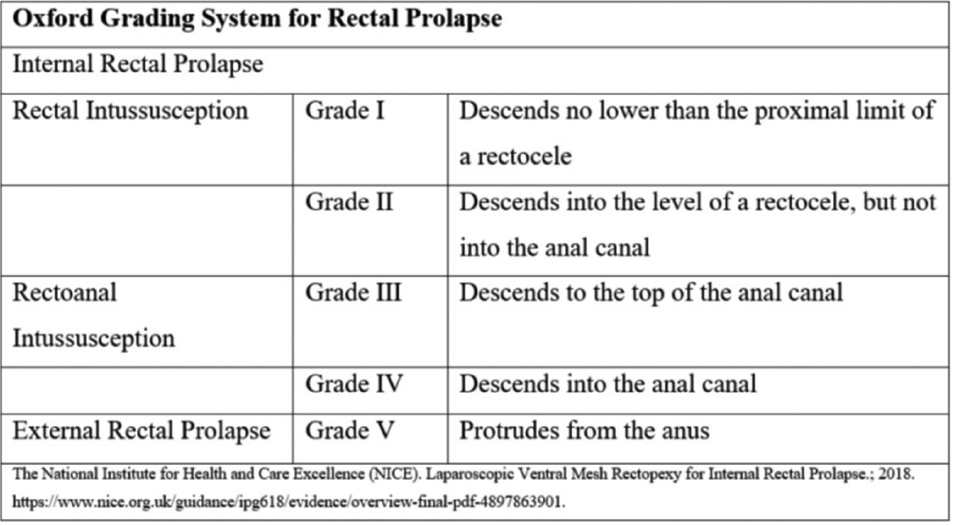
- Oxford grading system for rectal prolapse.
Surgical procedures for external RP are diverse. Nevertheless, the ultimate goal is to treat it, prevent its recurrence, restore defecation function, and prevent constipation or incontinence. Various abdominal and perineal procedures have been described for the management of RP (►Fig. 6). The perineal surgeries can be performed under regional anesthesia but have much higher long-term recurrence rates (ranging from 14% to 27% over 4 years) than the abdominal surgeries (ranging from 3% to 10%).4 Hence, they are now reserved only for high-risk patients who cannot withstand major abdominal surgery under general anesthesia. Among the commonly described perineal procedures are Thiersch's stitch, Delorme and Altemeier operations, and STARR. Thiersch's stitch refers to a circumferential “purse string” tightened stitch around the anal sphincter. As a standalone procedure, its role is limited only for the absolutely unfit patients, as it has a high recurrence rate. It is sometimes combined with the Delorme operation. Delorme procedure involves the dilation of the anus, separation of the mucosa from the sphincter and the muscularis propria, and the division of the mucosa together with the plication of the muscularis propria. It is considered a suitable procedure of choice for patients with smaller prolapses and recurrent RP after abdominal rectopexy.4 The recurrence rate after Delorme operation is 31%.1,5 Altemier operation (Perineal Procto-sigmoidectomy) involves full-thickness excision of the rectum and, if possible, a portion of the sigmoid colon. It is usually suited to manage external prolapse more than 5 cm long.4 Its recurrence rate is 24%.1,5

- Different surgical options for the management of rectal prolapse. The percentage figures denote individual recurrence rates. The year, numbers denote the years of origin of the individual operations. Lateral mesh rectopexy (Orr–Loygue rectopexy) was originally described by Orr in 1953 and was modified in 1984 by Loygue. Altemier's operation was first performed by Mikulicz in 1889 and was popularized by Altemier in the 1970s.
The abdominal approach is now considered the standard of care and is used whenever feasible. Essentially, the surgeries performed via the abdominal approach are collectively labeled as rectopexy. The word “rectopexy” alludes to the fixation of the rectum to the sacrum and is supposed to restore the physiological position of the rectum and thereby also correct the descent of the pelvic floor. The fixation can be achieved by simple stitching, stapling, or meshes. Abdominal procedures include suture rectopexy (Sudeck), mesh rectopexy, resection of redundant sigmoid colon (Frykman–Goldberg procedure: recurrence rate 13%),1,5 and a combined resection-rectopexy technique. Sutured rectopexy was first described by Sudeck in 1922. The operation includes a complete mobilization of the rectum down to the level of the levators. The rectum is then attached to the promontory by suture or staples. The dorsal mobilization induces fibrosis which helps to fixate and hold the rectum in place. The recurrence rate of Sudeck's suture rectopexy is 26%.1,5 In mesh rectopexy, a mesh or graft is used to achieve a broader fixation and induce more fibrosis. Used materials include fascia lata, synthetic meshes, and bio-meshes. The mesh can be placed anteriorly, posteriorly, laterally, or around the rectum. Anterior mesh rectopexy was described in 1952, by Ripstein. After complete mobilization of the rectum, a graft constructed out of the fascia lata was wrapped around the rectum and sutured to the promontory. Later instead of a fascia lata graft, synthetic meshes were used. Lateral mesh rectopexy (Orr – Loygue rectopexy) involves complete mobilization of the rectum anteriorly and posteriorly. Two mesh strips are sutured laterally to the rectum on both sides. The mesh strips are then sutured under tension to the promontory. Posterior mesh rectopexy (Wells), the subject of this study, entails the placement of a mesh around the posterior circumference of the rectum and then fixed to the promontory after complete mobilization of the rectum. The ventral third of the rectal circumference is spared to avoid fibrosis and stenosis by the shrinkage of the mesh. In 2004, D'Hoore et al published the results of a novel, autonomic nerve-sparing rectopexy technique. The dissection in this operation is strictly ventral in the recto-vaginal space down to the pelvic floor. There is no division of the lateral ligaments and, hence, no interruption of the autonomic nerve supply to the rectum. This has comparable recurrence rates with a much lesser incidence of de novo constipation or worsening of pre-existing constipation.6 Resection rectopexy (Frykman – Goldberg procedure) includes a sigmoid resection combined with a rectopexy, mostly a sutured rectopexy. The resection results in fibrosis around the anastomosis and the sacrum which leads to a rectal fixation to the sacrum and the colon lies in a straighter course which avoids torsion and sigmoidocele.
The abdominal surgeries can be performed by the open and laparoscopic approaches. In a randomized controlled study, laparoscopic rectopexy was found to have less operative pain, rapid recovery, and shorter post-operative hospital stay.7 Also, the surgical complications were significantly lower in comparison to open procedures. Laparoscopic approach is now considered the standard approach and is routinely recommended in all cases. Abdominal procedures involving sigmoid resection with or without rectopexy have reported recurrence rates of 2 to 5%. This technique also carries the risk of additional morbidity in the form of anastomotic leak and chances of incontinence following bowel resection, particularly in elderly individuals.8
Conventionally, mesh rectopexy involved the circumferential mobilization of the rectum up to pelvic floor with mesh placed ventrally or posteriorly. Complete rectal mobilization has been associated with autonomic nerve damage and disturbed recto-sigmoid motility leading to de novo constipation or worsening of pre-existing constipation.
The D'Hoore procedure or laparoscopic mesh ventral rectopexy (LMVR) has equivalent success rates and improved functional outcomes. It avoids the complications related to circumferential mobilization of rectum (de novo constipation) and colonic resection (anastomotic leak). Data suggest LMVR without posterior rectal mobilization as the surgical procedure of choice for RP as well as associated pelvic organ prolapse. The author described that the uniqueness of laparoscopic ventral rectopexy lies in the fact that mobilization is restricted to anterior rectum, thus leaving the autonomic innervation intact. Currently, this technique has gained widespread acceptance and has been proposed by many as the “standard of care” for the management of pelvic organ prolapse. The combined benefits of laparoscopic approach and ventral rectopexy have made the procedure safe and effective with minimal post-operative functional disturbance.9
Several studies have reported a recurrence rate of approximately 5% following LMVR. Most recurrences occur within the first 2 to 3 years. The risk of recurrence is similar to that reported for other abdominal procedures (2 – 9%).8,10 In the present study, no recurrence was found. The published recurrence rate of laparoscopic posterior rectopexy (Wells) is 11%.11 Hashida et al in their study on posterior rectopexy observed that the mean operative time of laparoscopic and open posterior mesh rectopexy was 127 and 83.6 minutes, respectively.10 The amount of blood-loss was negligible and 77 mL (range, 18 – 200 mL) with the laparoscopic and open approaches, respectively. The mean duration of hospitalization was 4.2 and 7.2 days, respectively (p < 0.05).10
Dyrberg et al in their study on posterior rectopexy observed that conversion to open surgery was done in 6.2%, the median operating time was 82 minutes (range 66 – 102 minutes) and median length of hospital stay was 2 days (range 2 – 5.7 days). Minor and major complications were seen in 5.3 and 14.8%, respectively.11
Hashida et al in their study observed that RP and fecal incontinence (evaluated using the Wexner score) diminished in all patients. Urinary incontinence also decreased postoperatively. There were no recurrences of RP.10
Dyrberg et al in their study observed that the 30-day mortality rate was 1.2%. Constipation or incontinence improved or disappeared in 65.2 and 74.4%, respectively. The cumulative recurrence rate was 11.1% after a median observation time of 2 years.11
The Indian population is predominantly vegetarian with high residue fiber as a major component of their diet. The sigmoid colon is particularly bulky and often redundant in this part of the world. There is, thus, a concern whether ventral rectopexy would be as effective in the treatment of CRP in this subset of patients as an alternative to resection rectopexy.
Laparoscopic posterior rectopexy appears to be a safe and effective surgical option for full-thickness RP, especially in Indian patients with bulky and redundant sigmoid colon. However, in view of small sample size and retrospective nature of this study, this needs to be validated by a larger study. Prospective randomized trials are warranted for level 1 evidence. A comprehensive review of literature touching various dimensions of the surgical therapy for RP is summarized (►Table 4).
| Authors [Ref.no.] | Journal (year of publication) | Type of study/no. of patients | Materials and methods | Conclusions |
|---|---|---|---|---|
| Bjerke and Mynster12 | Int J Colorectal Dis (2018) | Retrospective/1625 | 10 y data retrieved from Denmark's national data registry (2004 – 2014). | • Clear trend toward lap for primary and re-do repairs. • Highest recurrences in perineal procedures. |
| Flynn et al13 | Int J Colorectal Dis (2021) | Review, Meta-analysis | Medline, EMBASE, and Cochrane database searched for papers comparing robotic vs. lap repairs for RP | Longer operating times but shorter LOS with robotic vs. lap rectal prolapse repair. |
| Van der Schans et al3 | Tech Coloproctol (2018) | Clinical guidelines | Dutch Practice guidelines–A multidisciplinary drafting committee framed relevant questions, did literature search on electronic databases, and graded evidence, but in general–all statements made in “conclusions” column have low grades of evidence. | • LVR preferred procedure for ERP. • LVR preferred procedure for IRP grade 3 and 4 with compromised QOL or pain from SRU. • Contraindications: pregnancy, proctitis, pelvic floor dyssynergia, mentally unstable patients with IRP. • Heavy weight polypropylene-best material for rectopexy: no difference in erosion rates of synthetic vs. biological meshes. • Role of perineal procedures, when abdominal surgery contraindicated-adhesions such as in endometriosis, irradiation, diverticulitis, etc. • STARR is the preferred perineal procedure for IRP. • No difference between outcomes of STARR and Delorme. • STARR contraindicated in IRP with FI. • No difference in outcomes between Altemeier vs. Delorme. • No evidence favoring resection rectopexy over LVR. |
| Hyun et al14 | Ann Coloproctol (2019) | Retrospective study/293 | Results of Delorme and Thiersch compared in men vs. women. | • Men with RP younger and fitter than women. • Men had longer operating times, hospital stays, and more complications. • Recurrence rate of 7.8% (in males only 2.7%). |
| Gallo et al4 | Tech in Coloproctol (2018) | Review | Consensus statement of Italian society of colorectal surgery | • In expert hands results of perineal vs. abdominal surgeries for RP–similar. • For recurrent RP, perineal procedure contraindicated after abdominal mesh rectopexy. • After resection rectopexy (abdominal or perineal), perineal route can be used. • Short-term results of LVR vs. Delorme are similar. • Resection rectopexy results in reduced constipation vs. non-resectional rectopexy. • 60% laparoscopic, 20%open abdominal, and 20% perineal operations done for RP. • No differences between open & laparoscopic Sx for RP. • Currently, available evidence does not allow us to draw definitive conclusions. |
| Fu et al15 | Dis Colon Rectum (2017) | Retrospective cohort study/237 | 6 y study period, different clinical scenarios in which LVR was performed, compared with each other vis-à-vis recurrence of RP. | • Biologic grafts-lower recurrence. • Elderly patients with procidentia, poorer baseline continence, and prolonged pudendal nerve motor latency-higher recurrence. |
| Rautio et al16 | Tech Coloproctol (2016) | Retrospective multicenter Finnish study/52 males | 8 y study of LVR on males | • High overall recurrence-reoperation rate. • LVR should be modified or combined with additional abdominal/perineal methods, in males. |
| Laitakari et al17 | Tech Coloproctol (2021) | Retrospective cohort study/43 | 5 y multicenter Finnish study on re-do LVR. | • Re-do LVR safe and effective with acceptable recurrence and reoperation rates • Previous mesh left in situ in most and new one placed. |
| Tsunoda18 | J Anus Rectum Colon (2020) | Review | Current evidence base for laparoscopic vs. perineal procedures for RP and their results reviewed. | • Abdominal operations for young fit patients, perineal for old frail patients with comorbidities. • Laparoscopic had lower recurrence than perineal operations with comparable complication rates. • LRR and LVR improve constipation and prevent new onset constipation vs. other laparoscopic operations. • Optimum Sx not clear due to heterogeneity of available studies. • Individualized approach advised considering age, comorbidity, and underlying anatomical/functional disorders. |
| Madbouly and Youssef19 | J Laparoendosc Adv Surg Tech (2018) | Comparative study/74 | LVR vs. LPR. | • Operating time and shorter LOS in LPR. • High incontinence scores and age >70 y–predictors of bad postop continence. • LVR more suited in high constipation scores and abnormal perineal descent. |
| Emile et al20 | Colorectal Dis (2017) | Review/14studies/1,301 pts. (Abdominal rectopexy for IRP) |
PubMed, EMBASE, Cochrane central register searched for published studies between 2000 and 2015. | • Abdominal rectopexy–a good method for IRP. • VR had higher recurrence rates, lower complications, and better improvement of bowel symptoms than RR. |
| Emile et al21 | Colorectal Dis (2017) | RCT/50 | LVR vs. Delorme for complete RP. | • No significant difference between recurrence rates and symptom improvement. • Operating time shorter for Delorme but LOS longer. • Both operations at par at 18 mo follow-up. |
| Catanzarite et al22 | Dis Colon Rectum (2018) | Retrospective cohort/112 females with full thickness RP | Four subgroups formed: AR − POP, AR + POP, PR − POP, and PR + POP. | • POP associated with the higher recurrence rate. • Earlier recurrence in perineal repairs. • AR should be preferred in women with RP + POP. |
Abbreviations: AR, abdominal repair; ERP, external rectal prolapse; FI, fecal incontinence; IRP, internal rectal prolapse; lap., laparoscopy; LOS, Length of stay; LPR, laparoscopic posterior rectopexy; LRR, laparoscopic resection rectopexy; LVR, laparoscopic ventral rectopexy; POP, pelvic organ prolapse; PR, perineal repair; pro., procedures; QOL, quality of life; RCT, randomized controlled trial; RP, Rectal prolapse; RR, resection rectopexy; SRU, solitary rectal ulcer; STARR, stapled trans-anal resection of rectum; Sx, surgery; VR, ventral rectopexy
Conclusion
Laparoscopic posterior rectopexy can be safely performed in older patients to achieve early postoperative ambulation and significantly shorten the hospital stay. It may, therefore, be considered an effective treatment for CRP. As can be seen in this study, it is a very effective procedure, keeping in mind the recurrence rate. However, since the procedure entails posterior dissection and division of lateral ligaments of the rectum, incidences of de-novo constipation as well as worsening of pre-existing constipation are significantly high.
Conflict of Interest
None declared.
Funding
None.
References
- PROSPER: a randomised comparison of surgical treatments for rectal prolapse. Colorectal Dis. 2013;15(07):858-868.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic ventral rectopexy for rectal prolapse and symptomatic rectocele: an analysis of 245 consecutive patients. Colorectal Dis. 2013;15(06):695-699.
- [CrossRef] [PubMed] [Google Scholar]
- Management of patients with rectal prolapse: the 2017 Dutch guidelines. Tech Coloproctol. 2018;22(08):589-596.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus Statement of the Italian Society of Colorectal Surgery (SICCR): management and treatment of complete rectal prolapse. Tech Coloproctol. 2018;22(12):919-931.
- [CrossRef] [PubMed] [Google Scholar]
- What does the future hold for ventral rectopexy?: Functional outcome after laparoscopic posterior sutured rectopexy versus ventral mesh rectopexy for rectal prolapse: six-year follow-up of a double-blind, randomised single-centre study. EClinicalMedicine. 2019;16:2-3.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic ventral mesh rectopexy for complete rectal prolapse: a retrospective study evaluating outcomes in North Indian population. World J Gastrointest Surg. 2016;8(04):321-325.
- [CrossRef] [PubMed] [Google Scholar]
- It's the procedure not the patient: the operative approach is independently associated with an increased risk of complications after rectal prolapse repair. Colorectal Dis. 2012;14(03):362-368.
- [CrossRef] [PubMed] [Google Scholar]
- Practice parameters for the management of rectal prolapse. Dis Colon Rectum. 2011;54(11):1339-1346.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg. 2004;91(11):1500-1505.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness of laparoscopic posterior rectopexy for complete rectal prolapse: a cohort study. Int J Surg. 2019;72:109-114.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic posterior mesh rectopexy for rectal prolapse is a safe procedure in older patients: a prospective follow-up study. Scand J Surg. 2015;104(04):227-232.
- [CrossRef] [PubMed] [Google Scholar]
- One decade of rectal prolapse surgery: a national study. Int J Colorectal Dis. 2018;33(03):299-304.
- [CrossRef] [PubMed] [Google Scholar]
- Robotic versus laparoscopic ventral mesh rectopexy: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36(08):1621-1631.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Delorme-Thiersch operation outcomes in men and women with rectal prolapse. Ann Coloproctol. 2019;35(05):262-267.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for recurrence after laparoscopic ventral rectopexy. Dis Colon Rectum. 2017;60(02):178-186.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic ventral rectopexy in male patients with external rectal prolapse is associated with a high reoperation rate. Tech Coloproctol. 2016;20(10):715-720.
- [CrossRef] [PubMed] [Google Scholar]
- Redo ventral rectopexy: is it worthwhile? Tech Coloproctol. 2021;25(03):299-307.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of rectal prolapse in the laparoscopic era; a review of the literature. J Anus Rectum Colon. 2020;4(03):89-99.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic ventral rectopexy versus laparoscopic Wells rectopexy for complete rectal prolapse: long-term results. J Laparoendosc Adv Surg Tech A. 2018;28(01):1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Abdominal rectopexy for the treatment of internal rectal prolapse: a systematic review and meta-analysis. Colorectal Dis. 2017;19(01):O13-O24.
- [CrossRef] [PubMed] [Google Scholar]
- Laparoscopic ventral mesh rectopexy vs Delorme's operation in management of complete rectal prolapse: a prospective randomized study. Colorectal Dis. 2017;19(01):50-57.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of rectal prolapse after surgical repair in women with pelvic organ prolapse. Dis Colon Rectum. 2018;61(07):861-867.
- [CrossRef] [PubMed] [Google Scholar]







