Translate this page into:
“Clinicoradiological Score – Valuable Tool to Assess the Risk of Strangulated Small Bowel Obstruction in Tertiary Care Center”
*Corresponding author: Manikanta K.S., MBBS, MS (General Surgery), Department of General Surgery, Bangalore Medical College & Research Institute, Bangalore 560002, Karnataka, India. dr.manisurg@gmail.com
How to cite this article: Monisha G, Manikanta KS, Ranganath HH. “Clinicoradiological Score – Valuable Tool to Assess the Risk of Strangulated Small Bowel Obstruction in Tertiary Care Center”. Int J Recent Surg Med Sci. 2024;10:S1-6. doi: 10.1055/s-0043-1761416
Abstract
Objectives
Small bowel obstruction accounts for about 3% of the emergency laparotomies, hence there is a need for early recognition of the risk associated with strangulated small bowel obstruction. However, there is no single reliable tool for evaluating the bowel strangulation risk precisely and quickly, hence we sought to assess the specificity and sensitivity of a scoring system named “Clinicoradiological score” as a tool to assess the risk of strangulated small bowel obstruction in tertiary care center for early intervention.
Material and Methods
The study was an observational study conducted on 50 patients with clinical symptoms of small intestinal obstruction, diagnosed by CT and admitted in-patient basis at the general surgery department in the hospitals attached to Bangalore Medical College and Research Institute, Bengaluru from November 2017 to May 2019. In this scoring system, one point was given to each factor which includes pain duration (4 or more days), guarding, leucocyte count at least 10 ×109/L, C-reactive protein 75 mg/L or more, free fluid at least 500 mL (CT criteria), reduced wall contrast enhancement (CT criteria) leading to a maximum score of 6.
Based on standard treatment outcome, patients were grouped into three categories (conservative management, laparotomy without resection and anastomosis, and laparotomy with resection and anastomosis). Data was analyzed by descriptive statistics. The risk factors were compared between three patient groups and Chi-square test used for hypothesis testing. Sensitivity and specificity were evaluated for accuracy of score.
Results
Out of the six factors of Clinicoradiological score, five factors were found to be predictors (p-value <0.01), while history of pain more than 3 days did not have a significant p-value. In our study all 18 patients with score more than 3 had gangrenous changes and underwent resection. The p-value of the score was found to be significant.
Conclusion
Clinicoradiological score has been proved to be a valuable tool in predicting the risk of strangulated small bowel obstruction and ascertained the need for subsequent intestinal resection.
Keywords
Small bowel obstruction
Predictors
Strangulation
Resection and anastomosis
INTRODUCTION
Intestinal obstruction is defined as impairment to the passage of intestinal content and can be classified as either intrinsic or extrinsic, partial or complete, proximal, intermediate or distal (based on location). Small bowel obstruction accounts for about 3% of the emergency laparotomies.[1] Among the patients admitted with intestinal obstruction, 85% of partial small bowel obstructions resolve with nonoperative management, whereas operative intervention is done for those who fail conservative management and have evidence of vascular compromise, strangulation, or perforation.[2]
Around 5 to 15% of cases of sudden severe abdominal pain requiring hospital admission was diagnosed with mechanical obstruction. Small bowel obstruction is the cause of 80% of these cases of acute mechanical obstruction and approximately 6 to 13% of them had strangulated small bowel.[3] The prognosis of non-ischemic cases of small bowel obstruction is good with mortality rates as low as 2%, while prognosis of those with ischemia leads to mortality as high as 25%.[4]
Delayed surgery for a strangulated small bowel obstruction has a high mortality rate (8–25%). Though urgent CT might lead to an earlier decision to proceed to surgery, radiological signs alone are poor predictors (50–64%) of small bowel ischemia. CT has a sensitivity of 48% and a specificity of 83% in preoperative prediction of risk of bowel strangulation.[5] Hence, there is a need for early recognition of the risk associated with strangulation in small bowel obstruction. Our study was conducted with the aim of correlating the scoring with the outcome of patients presenting with a CT confirmed diagnosis of small bowel obstruction by measuring various clinical, laboratory, and radiological parameters.
Various studies have focused on different factors as a single best indicator of strangulation. The various factors studied were leukocytosis, fever, tachycardia, localized tenderness, feculent vomiting, raised WBC counts, raised lactate levels, X-ray findings, CT evaluation but none have proved to be effective as a single tool.[6]
Clinicoradiological score[7] comprises following six factors with a score of 1 for each.
Pain duration 4 or more days.
Guarding.
Leucocyte count at least 10 ×109/L.
C-reactive protein 75 mg/L or more.
CT criteria—peritoneal fluid at least 500 mL.
CT criteria—small bowel wall reduced contrast enhancement >3 mm, leading to a maximum total score of 6.
The scores were evaluated preoperatively at the time of admission and the patients were managed according to the guidelines, the study is an observational study. The correlation between the preoperative factors and outcome was assessed.
Objective of the Study
To estimate the risk of strangulated small bowel obstruction using clinicoradiological scores.
To determine the need for subsequent intestinal resection.
MATERIAL AND METHODS
Study design: Observational study.
Study period: November 2017 to May 2019.
Place of study: The study was conducted on in-patient basis at the general surgery department in the hospitals attached to Bangalore Medical College and Research Institute, Bengaluru.
Sample size: 50.
-
Inclusion criteria:
With clinical symptoms of intestinal obstruction.
Age >18 years.
Confirmed by radiological investigation (X-ray, CT).
Willing for informed written consent.
-
Exclusion
Large bowel obstruction.
Incarcerated abdominal wall hernia.
Immediate (within 1 month) postoperative ileus.
Inflammatory bowel disease.
Radiation-induced intestinal fibrosis.
Peritoneal carcinomatosis.
Methodology: The study was conducted in Victoria hospital and Bowring & Lady Curzon hospital on in-patients with clinical symptoms of intestinal obstruction confirmed by CT and were under evaluation.
Documentation included history, clinical evaluation involving physical examination with findings of fever and peritoneal signs (guarding, rebound), blood tests with at least white cell count, level of C-reactive protein and CT abdomen diagnosis of small bowel obstruction.
Based on clinical judgment, and guidelines, patients with suspected simple obstruction were managed conservatively with bowel rest, nasogastric decompression, and intravenous fluids. These conservatively managed patients were grouped under first category. Second category of patients include those with suspected complicated small bowel obstruction who underwent urgent laparotomy (with resection and anastomosis).
A third category was given to patients who underwent laparotomy but without resection anastomosis that included patients who underwent procedure like adhesiolysis, etc. Patients who underwent delayed laparotomy (more than 24 hours after admission) were excluded from the study.
Pain duration for 4 or more days, abdominal guarding, leucocyte count at least 10 ×109/L, C-reactive protein level 75 mg/L or greater, peritoneal fluid at least 500 mL and reduced bowel wall contrast enhancement were the six parameters which were considered preoperatively and given a score of 1 each.
After the initial management of the patient, the three groups were then correlated with the initial preoperative scores and results of the correlation were obtained. The sensitivity and specificity of individual factors were also obtained. The various causes of small bowel obstruction were also studied in relation to previous history of surgeries and the following results were obtained.
RESULTS
Fifty-six percent of the patients with history of pain less than 4 days were managed conservatively while 20% of the patients with pain less than 4 days underwent resection and anastomosis [Graph 1]. Less than 20% of patients with pain more than 3 days were managed conservatively while 52% of the patients with pain more than 4 days underwent resection and anastomosis with or without ileostomy [Graph 2].
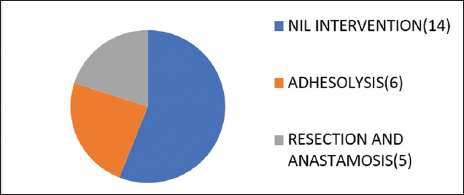
- Outcome for <4 days pain.
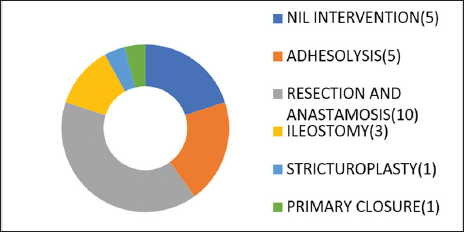
- Outcome ≥4 days pain.
Out of 33 patients with guarding, two patients were managed conservatively and 18 patients underwent resection and anastomosis. Seventeen patients who did not have guarding preoperatively, also belonged to the conservatively managed group [Graph 3].
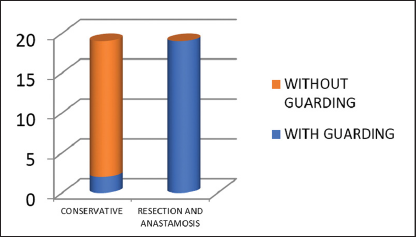
- Outcome for guarding.
Thirty patients had a raised CRP level, out of those 90% underwent laparotomy. Twenty-nine patients had counts more than 10 × 1010/L, 51.72% of those underwent resection and anastomosis and 13.79% were managed conservatively. Twenty-three patients had free fluid >500 mL [Graph 4] of which all 23 patients (100%) underwent laparotomy. Twenty-seven patients did not have free fluid more than 500 mL, of which 66.6% of them were managed conservatively.
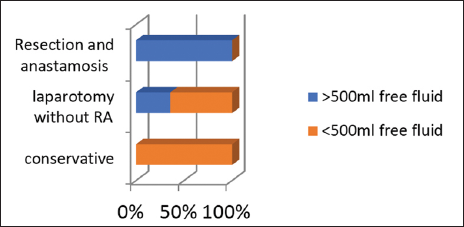
- Outcome for >500 mL free fluid.
Twenty-two patients had a reduction of CT small bowel contrast enhancement. 94.4% of them underwent resection and anastomosis. Specificity in our study is 100% and sensitivity is 94.4%.
The outcome of all the six factors of scoring system, with the percentage of patients who underwent resection and anastomosis and conservative management was tabulated [Table 1]. The specificity and sensitivity of each of the six factors were assessed [Table 2]. Out of the six factors, guarding, raised CRP and peritoneal free fluid had highest sensitivity of 100%, while specificity was highest for CT small bowel wall reduced contrast enhancement >3 mm, which was 100%.
Percentage RA |
Percentage conservative |
|
|---|---|---|
History of pain ≥4 d |
52% |
20% |
Guarding |
54.45% |
6% |
CRP level raised |
60% |
6.67% |
Leucocyte count 10 × 10(9)/L or greater |
51.72% |
13.79% |
Free fluid |
78.26% |
0% |
CT small bowel wall contrast enhancement >3 mm |
94.44% |
0% |
CRP, C-reactive protein
Specificity |
Sensitivity |
|
|---|---|---|
History of pain ≥4 d |
62.50% |
72.22% |
Guarding |
53.12% |
100% |
C-reactive protein level raised |
62.50% |
100% |
Leucocyte count 10 × 10(9)/L or greater |
56.25% |
83.33% |
Free fluid >500 mL |
84.38% |
100% |
Reduction in CT small bowel wall contrast enhancement >3 mm |
100% |
94.44% |
Chi-square value of the individual factors with resection and anastomosis was also sought. p-Value was significant for five out of the six factors [Table 3].
Chi-square |
p-Value |
|
|---|---|---|
History of pain lasting 4 d or more |
5.560 |
0.02a |
Guarding |
14.489 |
<0.001 |
C-reactive protein level raised |
18.750 |
<0.001 |
Leucocyte count 10 × 10(9)/L or greater |
7.410 |
<0.001 |
Free fluid |
33.016 |
<0.001 |
CT small bowel wall reduced contrast enhancement >3 mm |
45.791 |
<0.001 |
The correlation of outcome [Graph 5] with clinicoradiological score was done by categorizing the patients into three groups as already discussed. “p”-Value was calculated using Chi-square test and was found to be significant.
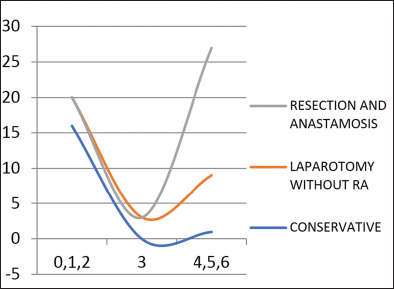
- Correlation of outcome with score.
Applying the clinicoradiological scores, about 17 of those who were managed conservatively based on the clinical findings had a score of 0,1,2. Out of the 14 patients who underwent laparotomy without resection and anastomosis, four patients had a score of less than 2, two patients had score of 3 and 8 of them had score above 3. Out of the 18 patients who underwent resection and anastomosis all of them had a score more than 3. Hence the scoring system had positive correlation with outcome studied [Table 4].
Outcome |
0,1,2 |
3 |
4,5,6 |
p-Value |
|---|---|---|---|---|
No laparotomy |
17 |
0 |
1 |
p <0.001 |
Laparotomy without resection and anastomosis |
4 |
2 |
8 |
|
Laparotomy with resection anastomosis |
0 |
0 |
18 |
We also sought to evaluate the various causes of small bowel obstruction in order to further extend our study for determining the risk of previous surgical history as an added risk factor for re-laparotomies.
The cause of small bowel obstruction was evaluated through various clinical and radiological methods and they are tabulated as shown [Table 5]. Simple adhesiolysis for adhesions is the commonest procedure performed. Adhesions are a major cause of morbidity in patients with history of previous surgery. Out of 50 patients who presented with obstruction, 25 have previous history of abdominal surgery. Out of 23 females, 11 females had history of gynecological surgery. Nine patients had history of laparotomy for GIT pathology. Five patients had history of appendicectomy [Graph 6]. These adhesions commonly occur after intraperitoneal intervention. However, the risk also depends on the type of surgery. The risk of adhesions is, however, more after gynecological and pelvic surgeries.
Diagnosis |
No |
Surgery |
|---|---|---|
Acute ileal intussusception |
1 |
RA |
Appendicular abscess |
1 |
Adhesiolysis |
Appendicular perforation with ileal perforation |
1 |
Primary closure |
Ileal gangrene |
7 |
6- RA 1-End ileostomy |
Ileal stricture |
1 |
Strictureplasty |
Ileal Tb |
5 |
2-RA 1-Ileostomy 2-Adhesiolysis |
Mesenteric ischemia |
3 |
2-RA 1-Nil |
Nil |
17 |
Nil |
Obstructed inguinal hernia |
3 |
2-RA 1-Adhesiolysis |
Obstructed umbilical hernia |
3 |
2-RA 1-Adhesiolysis |
Adhesions |
5 |
Adhesiolysis |
Sealed off perforation |
2 |
Adhesiolysis |
Strangulated obturator hernia |
1 |
Ileostomy |
Total |
50 |
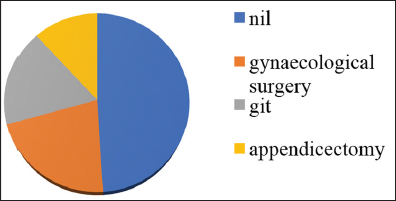
- Previous history of surgery.
In this study, [Table 5] maximum number of cases were adhesions for which adhesiolysis was done (11), followed by nonspecific inflammation and gangrene of the ileum (seven) for which resection and anastomosis was done in six and end ileostomy in one, followed by ileal tuberculosis (five), for which two patients underwent resection and anastomosis, one underwent resection and ileostomy, two underwent adhesiolysis. Obstructed hernia was seven, out of which inguinal was three, umbilical was three and obturator hernia was one. There was one case of acute ileal intussusception which underwent ileoileal anastomosis, one appendicular abscess which presented with small bowel obstruction which had dense adhesions for which adhesiolysis with abscess drainage was done.
Inference
From our study guarding, raised C-reactive protein levels, raised leucocyte counts, peritoneal free fluid >500 mL, CT small bowel wall reduced contrast enhancement >3 mm being predictors (p-value <0.01), while history of pain more than 3 days did not have significant p-value [Table 3].
History of pain in abdomen was one of the variables. Pain more than 3 days had a specificity of 62.50% and sensitivity of 72.22% with no significant p-value. Maximum duration of pain was 20 days. Minimum duration of pain was 1 day. Hence history of pain was not a reliable predictor in our study.
Guarding was one significant predictor of gangrenous bowel with specificity of 53.12% and sensitivity of 100%.
Raised C-reactive protein had specificity of 62.50% and sensitivity of 100% with a significant p-value.
Leucocyte count more than 10 × 109/L had a significant p-value with specificity of 56.25% and sensitivity of 83.33%.
CT findings: Free fluid more than 500 mL had a specificity of 84.38% and sensitivity of 100%. Reduction in CT wall thickness had a specificity of 100% and sensitivity of 94.44%.
DISCUSSION
Throughout the history, various studies were undertaken to manage strangulated bowel obstruction. In 1908, Scudder reviewed the cases operated for small bowel obstruction at Massachussets hospital from 1898 to 1907. Richardson in 1920 and Mclver in 1932 reviewed comparable series of cases. Monk in 1905 suggested that distended loop of bowel could be relieved by threading a tube to it. Wangsteen in 1931 suggested that applying constant suction to an inlying stomach or duodenal tube relieved mechanical obstruction. In 1934, Miller and Abbott along with Johnston devised a long flexible rubber tube to relieve the obstruction.[8] In 1967 to 1976, a study in University of Illinois[6] hospital among 238 patients of small bowel obstruction showed “classic” findings, i.e., leukocytosis, fever, tachycardia, and localized tenderness. The presence of any one or more of these findings mandates early operative intervention.
In 1981, a study[9] done at Montefiore hospital and North Central Bronx hospital among 405 patients showed that the presence of bowel strangulation shows a positive correlation with age (greater than 70 years), feculent vomiting, peristaltic sounds, and a white blood cell (WBC) count higher than 18,000/mm3. It shows no correlation with onset, localization or type of pain, duration of symptoms, temperature, tachycardia, or X-ray findings.
A retrospective study[5] was done in 1996 by Maglinte and Reyes, among 78 patients on the reliability of radiographs, which showed that the sensitivity of plain film radiography for revealing small-bowel obstruction was 69% (44/64), and its specificity was 57% (8/14). CT had sensitivities of 86% (24/28) and 82% (23/28).
Various other methods were used such as serum amylase, serum lactate (2.0 mmol/L or greater), intestinal fatty acid binding protein level, and low b value diffusion imaging for assessing the strangulation in small bowel obstruction cases. The study[10] by Sakamoto et al[10] in 2013, showed that serum I-FABP sensitively reflects bowel damage in SBO patients and seems to be a potential biomarker for detecting small-bowel ischemia yet it is a very late serum marker, hence cannot be used for early evaluation.
Following are the recent scoring system used for the assessment of acute small bowel obstruction. AGESS-SBO,[11] which consists of American Association for the Surgery of Trauma (AAST) anatomical score, physiological score, and co morbidities score was used for evaluation. This scoring system helps in prediction of outcome including mortality. A prediction model[12] was developed in 2017 for predicting the risk of strangulation in small bowel obstruction using following five factors, body temperature ≥38.0°C, positive peritoneal irritation sign, WBC count >10.0 × 109/L, thick-walled small bowel ≥3 mm, and ascites. This predictive model helps in the evaluation of small bowel obstruction severity and management of admitted patient.
A Diagnostic score[13] for acute small bowel obstruction was formulated based on the location of pain at diagnosis (LP + ) and without location of pain at diagnosis (LP–). Study showed that this Diagnostic score could be used for clinical diagnosis of ASBO without radiological or laboratory analyses, to reach a high diagnostic accuracy.
In this present clinicoradiological scoring system, all the variables have formerly been identified as independent predictors of strangulated bowel obstructions from literatures,[14–16] though from our study, history of pain more than 3 days was not proved to be a predictor. Study by Ha et al [17] proved CT finding for bowel vascular impairment had sensitivity of 34 to 48% and a specificity of 100%, while our study has 100% specificity for CT reduced contrast enhancement and sensitivity of 100% for free fluid in CT abdomen. In spite of the abovementioned various studies, the specificity and sensitivity of the factors in Clinicoradiological scoring prove to be significant, with a p-value <0.01. Hence the clinicoradiological scoring system proves to be a valuable tool in strangulated small bowel obstruction.
Limitations of Study
In this study sample size was small and needed a greater number of cases for precious conclusions. Since our institution is a tertiary care center, we mainly attended to cases which could not be managed under the primary or secondary level of health care; therefore, history of pain more than 3 days was not reliable and was also not found to be a predictor of strangulation in our study.
CONCLUSION
Clinicoradiological score has been proved to be quick and reliable tool in predicting the risk of strangulated small bowel obstruction and subsequent outcome.
Ethics approval
Approval was obtained from the Ethics Committee of Bangalore Medical College and Research Institute. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Conflicts of interest
None declared.
References
- The Clinical Significance of Adhesions: Focus on Intestinal Obstruction. Eur J Surg Suppl 1997:5-9.
- [Google Scholar]
- Sabiston Textbook of Surgery (21st). 2021. p. :1256.
- Prevalence, Causes and Management Outcome of Intestinal Obstruction in Adama Hospital, Ethiopia. BMC Surg. 2016;16:38.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Review of Small-bowel Obstruction: The Diagnosis and When to Worry. Radiology. 2015;275:332-42.
- [CrossRef] [PubMed] [Google Scholar]
- Reliability and Role of Plain Film Radiography and CT in the Diagnosis of Small-bowel Obstruction. AJR Am J Roentgenol. 1996;167:1451-5.
- [CrossRef] [PubMed] [Google Scholar]
- Critical Operative Management of Small Bowel Obstruction. Ann Surg. 1978;187:189-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinicoradiological Score for Predicting the Risk of Strangulated Small Bowel Obstruction. Br J Surg. 2010;97:1119-25.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Mechanical Obstruction of the Small Bowel—its Diagnosis and Treatment. N Engl J Med. 1940;222:611-22.
- [Google Scholar]
- Small Bowel Obstruction: The Role of Nonoperative Treatment in Simple Intestinal Obstruction and Predictive Criteria for Strangulation Obstruction. Surgery. 1981;89:407-13.
- [PubMed] [Google Scholar]
- Serum Intestinal Fatty Acid Binding Protein in Patients with Small Bowel Obstruction. Surg Sci. 2013;04:302-7.
- [Google Scholar]
- Validation of the Anatomic Severity Score Developed by the American Association for the Surgery of Trauma in Small Bowel Obstruction. J Surg Res. 2016;204:428-34.
- [CrossRef] [PubMed] [Google Scholar]
- A Prediction Model for Recognizing Strangulated Small Bowel Obstruction In: Sakai E, ed. Gastroenterology Research and Practice. 2018. p. :7164648.
- [Google Scholar]
- A Diagnostic Score for Acute Small Bowel Obstruction. Anticancer Res. 2021;41:1959-70.
- [CrossRef] [PubMed] [Google Scholar]
- Intestinal Ischemia in Patients in Whom Small Bowel Obstruction is Suspected: Evaluation of Accuracy, Limitations, and Clinical Implications of CT in Diagnosis. Radiology. 1997;205:519-22.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed Enhancement of the Bowel Wall: A New CT Sign of Small Bowel Strangulation. J Comput Assist Tomogr. 1996;20:379-81.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Mesenteric Ischemia: Diagnosis with Contrast-enhanced CT. Radiology. 1996;199:632-6.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation of Simple and Strangulated Small-Bowel Obstructions: Usefulness of Known CT Criteria. Radiology. 1997;204:507-12.
- [CrossRef] [PubMed] [Google Scholar]







