Translate this page into:
Comparative Study of Combination of Intralesional Triamcinolone Acetate with 5-Fluorouracil Versus Triamcinolone Alone in Treatment of Keloid

*Corresponding author: Dr. Sudeep Kumar, Department of general surgery, Era’s Lucknow medical college and hospital, Lucknow, India. sudeeprock@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar S, Kumar P, Asif S, Pandey S. Comparative study of combination of intralesional triamcinolone acetate with 5-fluorouracil versus triamcinolone alone in treatment of keloid. Int J Recent Sur Med Sci. 2024;10:22-29. doi: 10.25259/IJRSMS-2022-2-16
Abstract
Objectives
To assess various parameters of outcomes in the management of keloid by comparing the combination of 5-Fluorouracil (5-FU) with triamcinolone acetate and triamcinolone alone.
Materials and Methods
The present study was carried out as a prospective comparative study over a period of 24 months. A total of 70 diagnosed Keloids patients were included in the study, who were randomly divided into two equal groups: 35 patients were administered triamcinolone acetate and 5-FU (0.1 mL TA + 0.9 mL 5-FU) were classified as Group A (Triamcinolone acetonide [TAC] + 5-FU) while the remaining 35 (50.0%) patients were administered a 1 mL intralesional injection of triamcinolone acetate alone and were classified as Group B (TAC alone). Patients of both groups were administered the injections selected for them at a 3 week interval for 3–6 months. Patients and independent observers assessed the scar at each visit until the last follow-up. Assessment by patients was done for pain, itching, scar colour, stiffness, thickness and irregularity of the keloid on a 10-point scale, with higher values showing worse results. Assessment of scars by an independent observer was done on a similar scale, including vascularization, pigmentation, thickness and pliability.
Results
Initially vascularity, scar colour, vascularity and thickness of patients’ scars in both the groups assessed by the patient as well as the observer were comparable, which remained comparable up to the administration of the second dose. Thereafter, both patient and observer observed that the parameters of TAC + 5-FU administered patients were significantly lower than of those administered TAC alone. Assessment of irregularity in shape and pliability of scar was done only by the patient. Initially both irregularity and pliability of the patients in both groups were comparable, which remained comparable up to the administration of the third dose. Thereafter, the parameters of TAC + 5-FU administered patients were significantly lower than those administered TAC alone.
Conclusion
The findings in this study indicate that adjuvant 5-FU to TAC was more effective as compared to TAC alone.
Keywords
5-Fluorouracil
Keloid
Triamcinolone Acetate
INTRODUCTION
In modern-day literature, in early 19th century, Father of French Dermatology, Jean Louis Alibert, first described tumour-like scars, naming it “les cancroïdes de la peau”, when it became clear that these cicatricial tumours were non-cancerous, Alibert changed the name to “cheloïde” or “keloïde” in reference to the Greek word “chele” for crab’s claw and the suffix -oid meaning “like”.[1]
Keloids are caused by cutaneous injury and irritation, including trauma, insect bites, burns, surgery, vaccination, skin piercing, acne, folliculitis, chicken pox and herpes zoster infection. Although the exact pathology of keloid is not known, it is reported that keloid formation is the result of alterations in growth factors, collagen turnover, tension alignment, and genetic and immunological contributions.[2]
Keloids can lead to aesthetic and physical complaints along with functional impairment, causing psychogenic turmoil and severe depression in affected individuals.[3,4] Keloid is a condition known for its poor responsiveness to available treatment modalities and high recurrence rate, even after surgical excision.[5]
Currently, various forms of treatment for keloids exist; however, no single treatment has proven to be the most effective. Currently available treatment options are laser therapy, surgical excision, radiotherapy, silicone gel sheeting, flavonoids, adhesive tape support, pressure therapy, cryotherapy, 5-FU, Imiquimod, recombinant Transforming growth factor (TGF)-β3 and mannose-6-phosphate.[6,7]
Among the various treatment strategies available, intralesional corticosteroid injections are the most common and popular treatment approach.[8]
Triamcinolone acetonide (TAC) is considered the first line therapy for the treatment of early keloids.[9] It is the most commonly used intralesional corticosteroid for the treatment of keloids. It is supposed that corticosteroids, owing to their anti-inflammatory properties are helpful in suppressing the keloid scars.[10] Apart from this, corticosteroids also diminish collagen and glycosaminoglycan synthesis, inhibit fibroblast growth,[11] and enhance collagen and fibroblast degeneration.[9]
5-FU is a pyrimidine analogue that inhibits the thymidylate synthase enzyme, leading to suppression of nucleic acid synthesis and cell proliferation. It inhibits fibroblast proliferation, angiogenesis and TGF-β-induced collagen type I expression while increasing fibroblast apoptosis without necrosis.[12,13]
Intralesional injections of 5-FU can be used alone or in combination with other forms of therapy. Successful use of intralesional 5-FU injections alone or in combination with TAC injections has been reported.[14–16]
The present study was conducted to assess the adjuvant effect of 5-FU on intralesional TAC injection for the treatment of keloids.
MATERIAL AND METHODS
Study design
The present study was carried out as a prospective comparative study, at Department of Surgery, Era’s Lucknow Medical College & Hospital (ELMCH), over a period of 24 months.
Clearance for carrying out the study was obtained from the Institutional Ethical Committee, Era’s Lucknow Medical College & Hospital, Lucknow. Informed consent was obtained from all the patients.
Patients aged 18-60 years were included in the study. Patients with ulcerated and infected keloid, pregnancy and immunosuppression were excluded from the study.
The study included 35 patients in each group. All 70 clinically diagnosed patients with keloid were examined clinically, and necessary demographic details and clinical history, including previous treatments, etc. were recorded. Patients were randomly divided into two groups using the SNOSE (Sequentially numbered, opaque, sealed envelope) randomization technique as follows:
Group A patients were injected with a combination of triamcinolone acetate and 5-FU (0.1 mL TA + 0.9 mL 5-FU) intralesionally.
Group B patients were injected with 1 mL of triamcinolone acetate (40 mg) intralesionally.
Injections were given to both group of patients by a 27-gauge needle at an interval of 3 weeks for 3–6 months, with a 2-month follow-up. After each session and at final visit, patients and an independent observer blinded to treatment mode assessed the various parameters. Patients assessed the scar according to The Patient and Observer Scar Assessment Scale (POSAS) including pain, itching, scar colour, stiffness, thickness and irregularity of the Keloid. While observer assessed the scar, including vascularization, pigmentation, thickness and pliability. Before assessment, patients were educated on how to use the scale effectively.
Out of 70 patients enrolled in the study, 35 (50.0%) patients managed by intralesional injection of Triamcinolone acetate and 5-FU (0.1 mL TA + 0.9 mL 5-FU) were classified as Group A while rest 35 (50.0%) patients managed by 1 mL intralesional injection of triamcinolone acetate alone were classified as Group B.
The ages of patients enrolled in the study ranged from 18 to 60 years, mean age was 33.77 ± 12.36 years. Patients enrolled in Group A were older as compared to Group B (34.60±13.01 vs. 32.94 ± 11.81 years) but this difference was not found to be significant statistically. Out of 70 patients enrolled in the study, 37 (52.9%) were female and the rest were male. Though the proportion of females was higher in Group A as compared to Group B (57.1% vs. 48.6%), yet this difference was not found to be statistically significant. None of the patients had family history of Keloids. None of them had received any previous treatment. No clinical abnormality was detected during the clinical examination.
Keloids were observed in different parts of the body. Among overall patients, the most common site of keloids was the chest (18.6%) followed by abdomen (17.1%), ear lobe and wrist (11.4% each) and foot (8.6%). The site of keloids in both groups was comparable. The size of keloids of patients enrolled in the study ranged between 2 and 21 sq. cm, mean size was 10.24 ± 5.51 sq. cm. Though the size of keloids was higher in Group A (11.00 ± 5.82 sq. cm) as compared to Group B (9.49 ± 5.16 sq. cm), yet this difference was not found to be statistically significant.

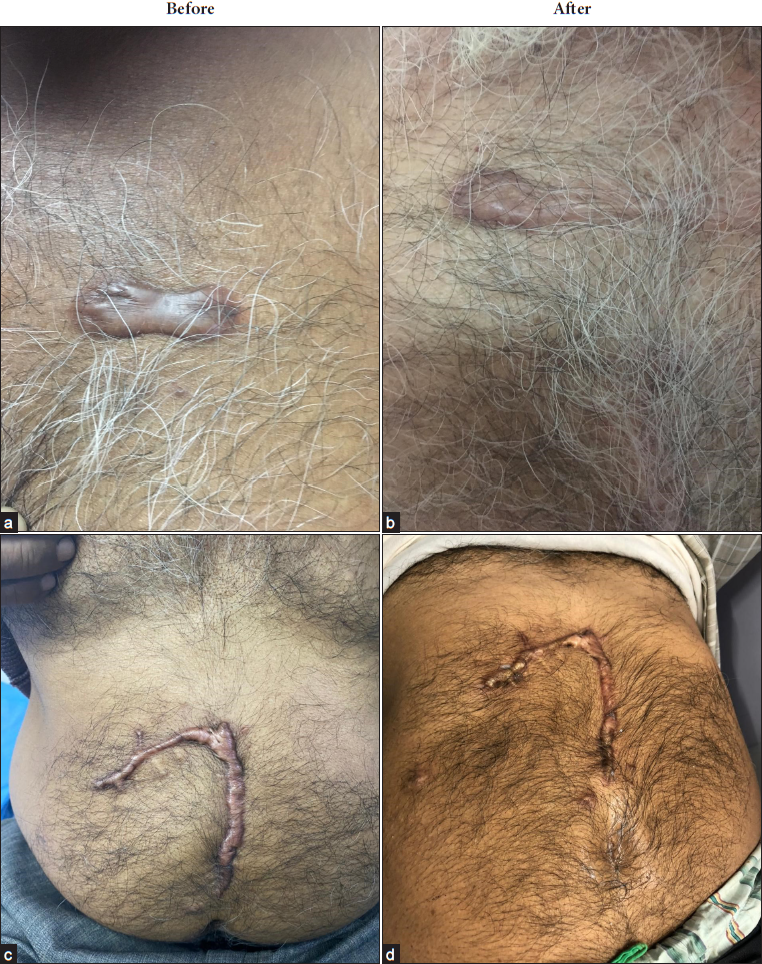
Evaluation procedures
All the patients enrolled in the study self-assessed six characteristics of keloids (Vascularity, Pigmentation, Thickness, Scar colour, Irregularity and Pain) at the initiation of treatment (first dose) and thereafter till the last dose (sixth dose) on a ten-point scale where score 1 indicates the normal and score 10 indicates the worst imaginable condition. The observer (clinician) also assessed the four characteristics (vascularity, pigmentation, thickness and pliability) of keloids using the same 10-point scale.
Statistical tools employed
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 Statistical Analysis Software. The values were represented in Number (%) and Mean ± SD.
RESULTS
Comparison of vascularity (patient & observer)
After the administration of the first and second doses, the assessment of the vascularity of patients from the above two groups was comparable. After the administration of third dose and thereafter until the sixth dose, Group A had significantly lower scores for patients’ assessments of vascularity. A statistically significant decline in the initial vascularity score assessed by patients was observed in both groups after administration of the last dose (sixth dose). Percentage decline in Group A was 24.17% while that in Group B was 14.14% [Figure 1].
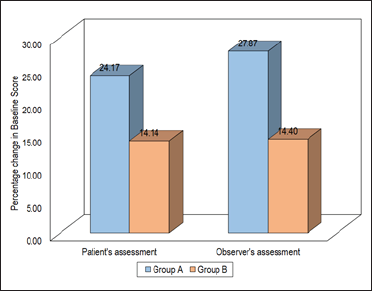
- Comparison of vascularity (patient and observer).
Similar findings were observed by the observer for the assessment of vascularity. A statistically significant decline in the initial vascularity score assessed by the observer was also observed after administration of the sixth (last) dose in both groups. In Group A, decline in the initial observer’s score for vascularity was 27.87% while in Group B it was 14.40% [Figure 1].
Comparison of pigmentation (patient and observer)
Pigmentation scores assessed by patients in the above two groups were comparable after administration of the first and second doses. At the administration of the third dose and thereafter until the sixth dose, patients’ assessed pigmentation scores in Group A were significantly lower than those in Group B. Similar findings were observed for the observers’ pigmentation scores.
A statistically significant decline in the initial pigmentation scores in both groups was observed after the administration of the last dose. The decline in patients’ assessed pigmentation score among the patients in Group A was 33.02% while that in Group B was 20.50%. The decline in the observer’s assessed initial pigmentation score in Group A was 33.29%, while that in Group B was 20.89% only [Figure 2].
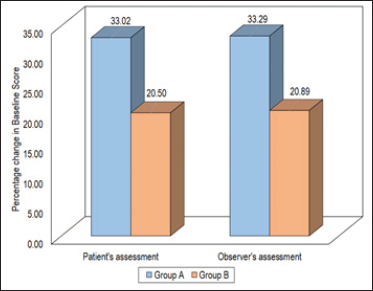
- Comparison of pigmentation (patient and observer).
Comparison of thickness (patient and observer)
Patients’ assessed thickness in the above two groups remained comparable until the administration of the third dose. After administration of the fourth dose and thereafter until administration of the sixth dose, patient’s assessed thickness in Group A was significantly lower than that in Group B.
The observers’ assessed thickness of patients in the above two groups remained comparable until the administration of the first two doses. After the administration of the third dose and thereafter until administration of the sixth dose, observer’s assessed thickness in Group A was significantly lower than that in Group B.
A significant decline in patients’ assessed as well as observers’ assessed initial thickness was observed in both groups after administration of the last dose. The percentage decline in patients’ assessed initial thickness score among patients in Group A was 22.43% while that in Group B was 7.86%. The percentage decline in the observer’s assessed initial thickness score among patients in Group A was 28.11% while that in Group B was 7.86% [Figure 3].
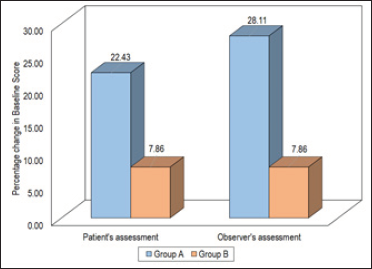
- Comparison of thickness (patient and observer).
Comparison of scar colour (patient’s assessment)
A significant decline in the initial scar colour score was observed in both groups. In Group A, the percentage decline in scar colour was 32.98% while that in Group B was 20.26% only [Figure 4].
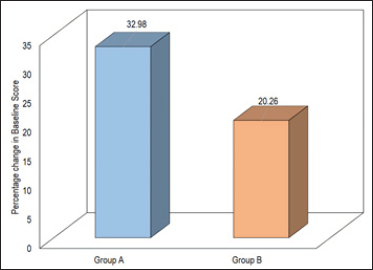
- Comparison of scar color (patient and observer).
Comparison of irregularity of shape (patient’s assessment)
A statistically significant decline in initial scores for irregularity of shape was observed in both groups. In Group A, the percentage change in initial scores for irregularity of shape was 24.53% while that in Group B was 10.99%.
Comparison of pliability (observer)
A statistically significant decline in initial pliability scores was observed in both groups. The percentage decline in initial pliability scores in Group A was 44.59% while that in Group B was 27.21% only.
Comparison of overall patient and observer assessment
Patients’ as well as observers’ overall assessment of the above two groups was comparable until the administration of the first two doses. After administration of the third dose and thereafter until administration of the last dose, the overall scores of Group A were found to be significantly lower than those of Group B.
A significant decline in the overall initial scores (patients’ assessment as well as observers’ assessment) in both groups was observed. A percentage decline in overall score was higher in Group A as compared to Group B as observed by patient (27.24% and 14.13%) as well as by observer (33.67% and 17.97%) [Figure 5].
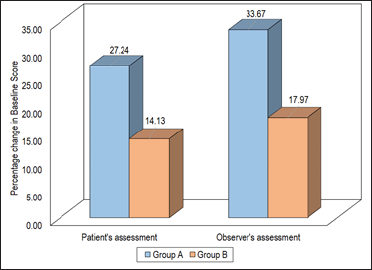
- Comparison of overall patient and observer assessment.
DISCUSSION
Keloids produce cosmetic disfigurement as well as functional impairment and can cause significant morbidity.[17] A variety of treatments such as pressure therapy, silicone gel sheeting, intralesional injections, cryotherapy, surgical manipulation, laser and radiotherapy are being used. However, no particular treatment is suitable or effective in all cases.[6]
Intralesional TAC is cost effective and practical and has become the first-line treatment in spite of many limitations. Corticosteroids seem to be effective as they diminish collagen and glycosaminoglycan synthesis, inhibit fibroblast growth,[11] enhance collagen and fibroblast degeneration,[8] and have powerful anti-inflammatory effect.[8] However, they cause local adverse effects such as dermal atrophy, telangiectasia and hypopigmentation. Common adverse effects of 5-FU use are skin erythema and ulceration that can be overcome by injecting a combination of 5-FU and TCA.[18] Therefore, the present study was proposed to study the efficacy of 5-FU adjuvant to TCA.
In the present study, 70 patients with keloids were included. The age range of patients was 18–60 years (mean age 34.60 years), and the majority of them were females (57.1%). These patients were randomly divided into two equal groups (35 patients each). In Group A, 35 patients were administered intralesional injections with a combination of triamcinolone acetate and 5-FU (0.1 mL TAC + 0.9 mL 5-FU) and the remaining 35 patients were administered intralesional injections of triamcinolone acetate (40 mg). Both groups were found to have comparable age and gender ratios.
In the present study, significant improvements in all the parameters in both groups were reported at the final assessment. At the final assessment, all the parameters showed significant improvement in TAC+5-FU patients as compared to TAC alone.
Darougheh et al.[5] compared TAC (10 mg) and TAC (4 mg) +5-FU (45 mg) efficacy for keloid management. They reported significant declines in height, erythema scores in both groups. Good to Excellent improvement was rated by patients in 80% of the TAC group and 100% of the TAC+FU group patients, while Good to Excellent improvement was reported by observers in 65% of TAC and 95% of TAC+5-FU patients.
Saha and Mukhopathyay[19] also compared TAC and TAC+5-FU combinations for the treatment of keloids. They found similar reductions in the volume of keloid in both groups. But TAC+FU had been found to be significantly effective for hyperpigmentation, pain at the injection site and superficial ulceration, while TAC was significantly effective for reducing overall pain.
Khan et al.[20] compared the efficacy of TAC+5-FU and TAC alone. They reported Good to Excellent results in a significantly higher proportion of TAC+5-FU as compared to the TAC alone group (84% vs. 50%).
All the previous studies have endorsed the superiority of TAC+5-FU over TAC alone in terms of clinical response, irrespective of differences in the method of assessment of response, duration of intervention, variability in dosage, and difference in patient characteristics. TAC+5-FU had been previously found to be safer and efficacious than TAC alone. One of the limitations of the study was the limited follow-up period during which complete resolution and/or recurrence could not be studied.
At the final assessment, both patient and observer scores showed significant declines from initial values. The percentage decline in initial overall score was higher in the TAC+5-FU administered group, as assessed by the patient as well as by the observer.
The findings in this study indicate that adjuvant 5-FU to TAC was more effective as compared to TAC alone.
Acknowledgement
None.
Declaration of patients consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
REFERENCES
- The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. 2020;8:360.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Keloid scarring: Understanding the genetic basis, advances, and prospects. Arch Plast Surg. 2012;39:184-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433-38.
- [CrossRef] [PubMed] [Google Scholar]
- The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61:1049-58.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional triamcinolone alone or in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. Clin Exp Dermatol. 2009;34:219-23.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-25.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Keloids: Current and emerging therapies. Scars Burn Heal. 2020;6:2059513120940499.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histomorphologic changes in keloids treated with Kenacort. J Trauma. 1995;38:299-302.
- [CrossRef] [PubMed] [Google Scholar]
- International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-71.
- [CrossRef] [PubMed] [Google Scholar]
- Scar treatments: preclinical and clinical studies. J Am Coll Surg. 2008;206:719-30.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of human keloid fibroblast growth by isotretinoin and triamcinolone acetonide in vitro. Ann Plast Surg. 1994;33:401-5.
- [CrossRef] [PubMed] [Google Scholar]
- Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. 2018;19:E711.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intralesional 5-fluorouracil in keloid treatment: A systematic review. Acta Derm Venereol. 2015;95:778-82.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional 5-fluorouracil as a treatment modality of keloids. Dermatol Surg. 2004;30:54-56.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: Randomised control trial. Burns. 2019;45:69-75.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: a systematic review and meta-analysis. Int Wound J. 2017;14:480-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433-8.
- [CrossRef] [PubMed] [Google Scholar]
- Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387-96.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A comparative clinical study on role of 5‐Flurouracil versus triamcinolone in the treatment of keloids. Indian J Surg. 2012;74:326-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional triamcinolone alone and in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. J Pak Med Assoc. 2014;64:1003-7.
- [PubMed] [Google Scholar]







