Translate this page into:
Evaluation of Antibiotic Sensitivity in Deep Neck Space Infections
How to cite this article: Singhal G, Nayak P, Padiyar V, Sen K, Shrivastava SS. Evaluation of antibiotic sensitivity in deep neck space infections. Int J Recent Sur Med Sci, doi: 10.1055/s-0043-1761508
Abstract
Background
Deep neck space infections (DNIs) are a major medical concern in the Indian community. Owing to the complex anatomy of the neck spaces and their communication with each other, accurate diagnosis becomes challenging. A thorough knowledge of the anatomy as well as the microbiological profile and antibiotic sensitivity is imperative to institute the appropriate surgical and medical management to the patient. Due to the advent of broad-spectrum antibiotics, the incidence of these infections have declined considerably over the last couple of decades. However, due to the extensive and unregulated use, the incidence of antibiotic resistance has also been increasing at an alarming pace.
Materials and Methods
This cross-sectional observational study was conducted in the Department of Otorhinolaryngology at a tertiary care government hospital in an urban area. All patients who presented to the OPD or emergency over a period of 18 months and who fulfilled the eligibility criteria were included in the study. Pus was collected from the abscess, aseptically by needle aspiration using wide bore (18G) needle and transported under all aseptic measures within 24 hours for culture and sensitivity, KOH mount, and detection of AFB. Antibiotic sensitivity testing was done using the Kirby Bauer disc diffusion method and E-test.
Results
Staphylococcus aureus as the most common infective organism followed by MRSA in the pediatric age group and Klebsiella pneumoniae in adults.
Conclusion
Primary knowledge of individual antibiotic sensitivity is imperative to ensure prompt and adequate treatment of the patient with higher chances of complete resolution, concomitantly minimizing the risk of resistance. Inadequate and delayed treatment may lead to swift progression of the disease with significant morbidity and mortality.
Keywords
Deep neck space infection
Antibiotic sensitivity
resistance
Introduction
Deep neck space infection (DNI) is defined as the infection of the deep fascia enclosing potential spaces of the neck. They remain a major medical concern in the Indian community. Owing to the complex anatomy of the neck spaces and their communication with each other, accurate diagnosis becomes challenging. Also, the close proximity of this space to the airway and various communicating pathways to other regions can lead to critical complications such as empyema, mediastinitis, pericarditis, jugular-vein thrombosis, septic shock, respiratory distress, disseminated intravascular coagulation and dissemination[1] unless diagnosed and managed promptly.[2,3]
A thorough knowledge of the anatomy as well as the microbiological profile and antibiotic sensitivity is imperative to institute the appropriate surgical and medical management to the patient. Surgical management by incision and drainage and immediate airway management remains the mainstay of treatment.[4] However, concomitant therapy by aggressive and prompt use of antibiotics have been reported to significantly reduce the morbidity in patients.[5]
Due to the advent of broad-spectrum antibiotics, the incidence of these infections have declined considerably over the last couple of decades. However, due to extensive and unregulated use, the incidence of antibiotic resistance has also been increasing at an alarming pace.[6] Therefore, primary knowledge of the antibiotic sensitivity is imperative to ensure prompt and adequate treatment of the patient with higher chances of complete resolution while minimizing the risk of resistance. Inadequate and delayed treatment may lead to swift progression of the disease with significant morbidity and mortality.
This study was conducted to determine the sensitivity of the commonly used antibiotics in the primary treatment of deep neck space infections.
Materials and Methods
This cross-sectional observational study was conducted in the Department of Otorhinolaryngology at a tertiary care government hospital in an urban area. A total of 82 patients who presented to the OPD or emergency over a period of 18 months and who fulfilled the eligibility criteria were included in the study.
Patients of all ages and gender presenting with deep neck space infections confirmed either clinically or radiologically were included in the study. Patients excused from the study comprised the following:
-
Patients with superficial neck space infections
-
Patients who had received antibiotics for more than 48 hours or had taken incomplete treatment
-
Those presenting with infections secondary to traumatic or iatrogenic neck wounds were excluded from the study.
Ethical clearance was obtained from the institutional ethics committee prior to the commencement of the trial and appropriate written, informed consent was taken from each patient who participated in the study.
All eligible patients were subjected to a thorough history taking, followed by an extensive clinical examination. Patients below 18 years of age were included in the pediatric age group. while those who were 18 years or above were included in the adult age group. Blood samples were drawn and sent for the assessment of pathological, biochemical, and microbiological parameters. Radiological imaging techniques were such as X-ray neck (soft tissue-lateral view), ultrasonography, and contract-enhanced CT were done wherever deemed necessary.
Pus was collected aseptically by needle aspiration using a wide bore (18G) needle and transported to the Department of Microbiology under all aseptic measures within 24 hours for culture and sensitivity, KOH mount, and detection of AFB. Antibiotic sensitivity testing was done using the Kirby Bauer disc diffusion method and E-test.
The patients were then subjected to incision and surgical drainage of the abscesses, followed by daily debridement and dressing under general anesthesia. Empirical therapy was commenced for all patients with broad-spectrum intravenous antibiotics. Intravenous amoxicillin and clavulanic acid, metronidazole and amikacin were started for all patients pending the future sensitivity reports.
The data obtained were collated and analyzed using statistical package for the social science (SPSS, version 21.0). Statistical analysis was done using paired and unpaired t-test for quantitative variables and Chi-square test for qualitative ones. Quantitative data were expressed as mean ± SD, whereas qualitative data are expressed in terms of frequency and percentage. The confidence interval was set to 95% and the margin of error accepted was 5%. P < 0.05 was considered statistically significant.
Results
In our study, 82 patients presented to our institution with DNI with a mean age of 31.45 years. Of the 82 patients, 22 were in the pediatric age group, whereas 60 patients were adults. Fifty-four patients were males and 28 were females, and 25 patients out of 60 patients of the adult age group were diagnosed with type II diabetes mellitus.
On culture sensitivity, bacteria were isolated in 54 cases. In the pediatric age group, Staphylococcus aureus was the most common bacteria isolated in eight cases, followed by methicillin-resistant Staphylococcus aureus (MRSA) in seven cases, Escherichia coli and Klebsiella species in two cases each, and Streptococcus viridans in one case.
Similarly in the adult age group, Staphylococcus aureus was the most common bacteria isolated (11 cases), followed by Klebsiella species in 9 cases. Other bacteria isolated were E. coli, MRSA, S. viridans, Enterococcus faecum, Enterococcus species, Streptococcus species, Pseudomonas species, and Klebsiella pneumoniae. Poly-microbial infections were seen in two cases who were diabetics.
Staphylococcus aureus was sensitive to vancomycin and cefuroxime in a 100% of cases in both pediatric and adult age groups. In the pediatric age group, S. aureus was also sensitive to oxacillin in 100% cases. Staphylococcus aureus was also sensitive to tetracycline, erythromycin, ciprofloxacin, and cotrimoxazole [Figure 1]. Similarly, MRSA was sensitive to vancomycin in 100% patients followed by cotrimoxazole, tetracycline, and erythromycin. However, complete resistance was seen to amoxycillin clavulanate, ampicillin, and other drugs of the amino penicillin group [Figure 2].
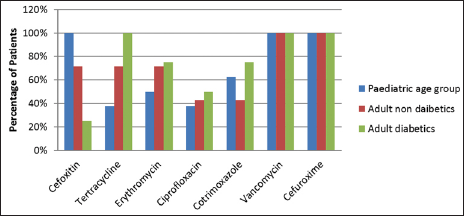
- Distribution of patients according to antibiotic sensitivity for Staphylococcus aureus.
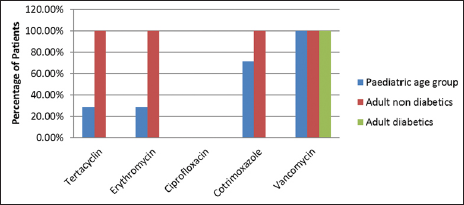
- Distribution of patients according to antibiotic sensitivity for MRSA.
Klebsiella species was the second most common microorganism isolated and it was sensitive to amikacin and piperacillin tazobactum in 100% patients of both adult and pediatric age groups. Sensitivity to gentamycin was seen in 100% patients in both pediatric age group and adult diabetics, while it was 66.67% in adult nondiabetics. It was also sensitive to ciprofloxacin in 100% cases in the pediatric age group. Klebsiella species also showed sensitivity to cotrimoxazole, cefotaxime, ceftriaxone, and ceftazidime. A similar trend was observed for Klebsiella pneumoniae, it was sensitive to gentamycin, ciprofloxacin, amikacin and piperacillin tazobactum [Figure 3].
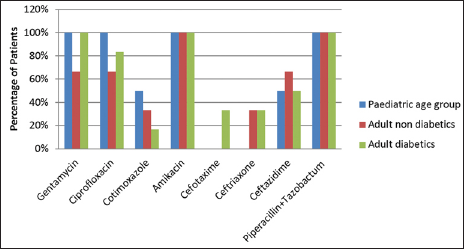
- Distribution of patients according to antibiotic sensitivity for Klebsiella.
Streptococcus did not show resistance to any of the antibiotics and it was 100% sensitive to penicillin, ampicillin, tetracycline, erythromycin and linezolid.
Escherichia coli had 100% sensitivity for piperacillin tazobactum and meropenem among pediatric and adult age groups, while it had sensitivity of 100% for gentamycin in the pediatric age group and adult nondiabetics but not for adult diabetics. It was also sensitive for cotrimoxazole, amikacin, ceftriaxone, and ceftazidime [Figure 4].
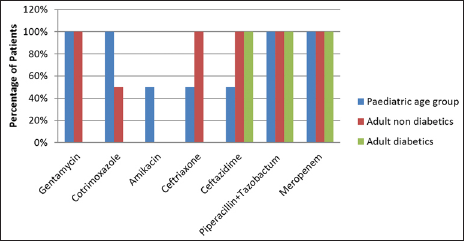
- Distribution of patients according to antibiotic sensitivity for Escherichia coli.
One case of Pseudomonas spp. was seen in a patient who was an adult diabetic and it was sensitive to all antibiotics, oxacillin, gentamycin, ciprofloxacin, amikacin, ceftazidime, piperacillin tazobactum, piperacillin, and meropenem.
Enterococcus faecium and Enterococcus spp. were isolated in one case each, who were adult nondiabetics. Enterococcus faecium was sensitive only to vancomycin and linezolid, while Enterococcus spp. was sensitive to ampicillin, gentamycin, vancomycin, piperacillin tazobactum, and linezolid.
Discussion
DNI is essentially suppuration in the potential spaces of the neck. Multiple layers of deep cervical fascia encases the contents of the neck to form the potential head and neck spaces, condensation of which forms the fascial planes. These spaces contain adipose tissue and majority of the major neuro-vasculature supplying the head and neck region.
Because DNI is a potentially life-threatening infection, immediate empirical antibiotic therapy has been recommended, besides definitive surgical and airway management, to decrease the associated morbidity and mortality.[7][Table 1]. Broad-spectrum antibiotics have been used extensively over the past couple of decades as most cases have been found to involve gram-positive cocci, gram-negative rods with or without anaerobes.[8] Overall, penicillin with or without metronidazole and clindamycin has been found to be highly efficacious. Combined therapy of penicillin and metronidazole covers both aerobes and anaerobes.[7,9] Antibiotic coverage may be expanded in cases of antecedent otologic or sinonasal infections or nosocomial infections in which Pseudomonas is more common. As rates of MRSA have increased in the community especially in children below 2 years of age, clindamycin has also been widely considered the initial therapy of choice in recent times. Cheng et al conducted a study on 178 pediatric patients with retropharyngeal or parapharyngeal infections and found MRSA to be the commonest etiology. The MRSA cultures were found to be sensitive to clindamycin and trimethoprim/sulfamethoxazole in all but one case, which demonstrated resistance to clindamycin and sensitivity to trimethoprim/sulfamethaxozole.[10,11]
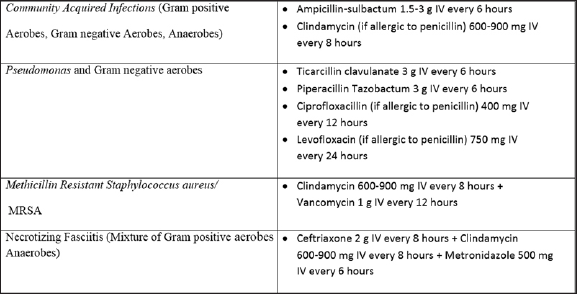
Ampicillin sulbactum has been recommended in cases of resistance to penicillin G and clindamycin. Fluoroquinolones, third-generation cephalosporins and vancomycins have been used as alternatives in case of resistant strains. This has in fact greatly reduced the morbidity in DNI. It has also prevented infections such as peri-tonsillar abscess, acute tonsilitis, and upper respiratory infections from progressing to DNI.[1]
On the downside, however, the injudicious use of these antibiotics for other minor conditions as well as for DNI without subsequent culture and sensitivity has led to meteoric rise in resistant strains. Clindamycin resistance has been observed in 5% to 10% of MRSA-related pediatric patients.[10] Maharaj et al observed that clindamycin may no longer be considered a first-line antibiotic in DNI, as the current resistance rates among Bacteroides fragilis strains worldwide had reached 20% to 50%.[9]
In the study conducted by Shah et al, they observed that S. aureus was resistant to amoxicillin in 31.3% of cases, while 93.8% of patients were sensitive to amoxicillin/clavulanic acid.[12] Walia et al found that resistance to penicillin by gram-positive aerobes was in (38.88%) of the isolates. S. aureus was seen to be resistant to penicillin and erythromycin in five cases (71.42%). 100% susceptibility was noted to gentamicin, ciprofloxacin, and cephotaxime. Cephotaxime along with ciprofloxacillin was found to be effective against the entire gram-positive spectrum isolated in this study.[13] In our study, S. aureus was sensitive to vancomycin and cefuroxime in 100% cases, while it was less sensitive to other drugs. In the pediatric age group, 100% cases of S. aureus were also sensitive to oxacillin. Similarly, MRSA was sensitive to vancomycin in 100% of patients.
Similarly, Rega et al observed that Staphylococci showed a 27.3% susceptibility rate to penicillin. Staphylococci also showed susceptibility to ampicillin (41.2%), cefazolin (70.0%), ciprofloxacin (95%), clindamycin (89.5%), erythromycin (75%), levofloxacin (84.2%), and vancomycin (100%).[14]
In our study, Streptococcus was sensitive to penicillin, ampicillin, tetracycline, erythromycin and linezolid in all cases similar to Rega et al who observed that Viridans streptococci showed an 87.1% susceptibility rate to penicillin. Viridans streptococci also showed a high susceptibility to ampicillin (98.4%), cefazolin (100%), ciprofloxacin (100%), clindamycin (86.3%), erythromycin (83.4%), levofloxacin (98.6%), and vancomycin (100%).[14] Shah et al,[12] in their study, reported Streptococcus viridans was resistant to amoxicillin in 34% of cases and amoxicillin with clavulanic acid in 4.3% of cases while it was sensitive to all other drugs.[12] Walia et al reported that S. viridans showed resistance to penicillin. Erythromycin and gentamycin in one isolate (25%), whereas 100% susceptibility was noted to ciprofloxacin and cephotaxime.[13]
Fating et al conducted a study on 26 patients with DNI and found that all aerobic strains were sensitive to gentamycin, vancomycin, imipenam, and linezolid, whereas 80% of the strains were sensitive to penicillin G, amoxicillin, and amoxycillin and clavulinic acid. Also, 20% of the isolated strains were resistant to penicillin G, amoxycillin, and amoxicillin and clavulinic acid. Next, 10% were resistant to doxycycline and cefixime.[15]
In this study, the antibiotic susceptibility of gram-negative microorganisms was seen predominantly with Amikacin. E. coli and Klebsiella were found 100% susceptible to Amikacin, whereas Pseudomonas was 100% resistant to amikacin, but was sensitive to cefotaxime, cefuroxime, and ciprofloxacin. In a similar study, Shah et al[12] found that E. coli was resistant to ceftriaxone in 33.3% of the cases, whereas carbenicillin, amikacin, and moxifloxacin were most sensitive (66.7%) and imipenem was intermediately sensitive in all (3%) cases. However, they reported 100% resistance with amoxicillin and amoxicillin with clavulanic acid, while carbenicillin showed 16.7% resistance only.[12]
In a study by Rijal et al, the highest sensitivity was seen for meropenem (73.58%), cefoperazone sulbactam (69.36%), and oxacillin (66.67%), while resistance was observed to aminopenicillin antibiotics (54.29%), gentamycin (52.27%), and ampicillin-sulbactam (37.89%).[16]
The results of Boyanova et al’s research showed that the resistance of etiological bacteria in DNI to amoxicillin was 26.7%, while that of clindamycin and metronidazole against gram-negative anaerobes were 5.4% and 2.5%, respectively, and against gram-positive in 4.5% and 58.3%, respectively. They deduced that metronidazole and clindamycin were the most effective options for the management of predominantly anaerobic infections.[17]
Devi et al observed that most cases (33, 82.5%) were sensitive to amoxycillin with clavulanic acid. Most cases responded to third-generation cephalosporins (87.5%), piperacillin with tazobactam (95%), ciprofloxacin (90%), amikacin (90%), meropenem (95%), cotrimoxazole (90%), colistin (90%), and tigecycline (90%).[18]
We see, therefore, that the use of antibiotics has proven to be a double-edged sword in the management of DNI. On the one hand, there has been a drastic fall in the morbidity and mortality due to the empirical use of these chemotherapeutic agents. On the other hand, there is a steady rise in the resistance of microorganisms to the first-line antibiotics.
Conclusion
The bacteriological study of DNI showed Staphylococcus aureus as the most common infective organism followed by MRSA in the pediatric age group and Klebsiella pneumoniae in adults. Other organisms isolated were Klebsiella, Streptococcus viridans, E. coli, Pseudomonas spp., and Enterococcus spp.
It is alarming to note that the commonly used first-line drugs such as broad-spectrum penicillins and aminopenicillins show rapidly increasing resistance in cases of DNI. Although susceptibility was seen to be present to fluoroquinolones, a significant proportion of the cases were seen to be resistant to this group of antibiotics as well. Indiscriminate use of antibiotics to empirically treat minor ailments should be discouraged immediately in view of the precarious situation we find ourselves in. Considering the rate of morbidity and fatality in this disease, it would be a dire situation indeed if despite the availability of a whole plethora of medications, the morbidity and the mortality of this condition could not be addressed effectively.
Continued care must be taken to ensure efficacy of antibiotic medications. Careful use of systemic antibiotics, prompt culture, and sensitivity testing with early debridement and surgical clearance of the disease when applied judiciously are extremely effective treatment modalities for DNI. The patients should also be given medications for the complete duration as per the protocol and subjected repeated sensitivity testing periodically over the course of the disease. This will not only help in minimizing the complications but also in preventing the emergence of resistant strains.
Funding
None.
Conflict of interests
None declared.
References
- Deep neck infection. Otolaryngol Clin North Am. 2008;41:459-83. vii
- [CrossRef] [PubMed] [Google Scholar]
- Deep neck abscesses: study of 101 cases. Rev Bras Otorrinolaringol (Engl Ed). 2017;83:341-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Current trents in pathogenesis, management, bacteriologyand antibiotic resistance in deep neck space infection: An institutional review. Ann Indian Acad Otorhinolaryngol Head Neck Surg.. 2020;4:5-9.
- [Google Scholar]
- Deep neck space infection - a retrospective study of 270 cases at tertiary care center. World J Otorhinolaryngol Head Neck Surg. 2016;2:208-13.
- [CrossRef] [Google Scholar]
- Predisposing factors of complicated deep neck infection: an analysis of 158 cases. Yonsei Med J. 2007;48:55-62.
- [CrossRef] [PubMed] [Google Scholar]
- Deep neck infection: a review of 130 cases in southern china. Medicine (Baltimore). 2015;94:e994.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Deep Neck Space Infections In: Oliver E, Gillespie M, eds. Cummings Otolaryngology Head & Neck Surgery. Philadelphia: Mosby Elsevier; 2010. p. :201-8.
- [Google Scholar]
- Deep neck abscess: an analysis of microbial etiology and the effectiveness of antibiotics. Infect Drug Resist. 2008;1:1-8.
- [CrossRef] [Google Scholar]
- Deep neck space infections: a case series and review of the literature. Clin Med Insights Ear Nose Throat 2019;12;
- [Google Scholar]
- Children with deep space neck infections: our experience with 178 children. Otolaryngol Head Neck Surg. 2013;148:1037-42.
- [CrossRef] [Google Scholar]
- The increased risk of community-acquired methicillin-resistant Staphylococcus aureus in neck infections in young children. Curr Infect Dis Rep. 2012;14:119-20.
- [CrossRef] [Google Scholar]
- Aerobic microbiology and culture sensitivity of head and neck space infection of odontogenic origin. Natl J Maxillofac Surg. 2016;7:56-61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. J Maxillofac Oral Surg. 2014;13:16-21.
- [CrossRef] [Google Scholar]
- Microbiology and antibiotic sensitivities of deep neck space infections. J Oral Maxillofac Surg. 2006;62:25-6.
- [CrossRef] [Google Scholar]
- Detection of bacterial flora in orofacial space infections and their antibiotic sensitivity profile. J Maxillofac Oral Surg. 2014;13:525-32.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bacteria pattern, results of antibiotic sensitivity test, and complications of deep neck abscess patients in Dr. Soetomo General Hospital. Biomolecular Health Sci J.. 2018;1:124.
- [Google Scholar]
- Anaerobic bacteria in 118 patients with deep-space head and neck infections from the University Hospital of Maxillofacial Surgery, Sofia, Bulgaria. J Med Microbiol. 2006;55:1285-9.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical analysis of infections of deep neck space with respect to bacteriology and antibiotic sensitivity. IOSR J Dent Med Sci. 2019;18:49-54.
- [Google Scholar]








