Translate this page into:
Evaluation of Efficacy of Heparin in Patients with Cellulitis
Address for correspondence Rajat Choudhari, Department of General Surgery, Kasturba Medical College Mangalore, Manipal Academy of Higher Education, Manipal 575001, Karnataka, India (e-mail: rajatc10@gmail.com).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
Heparin is useful in inflammatory conditions for amelioration of symptoms by altering the microcellular environment. This study was aimed at evaluation of the role of heparin in patients with cellulitis on antibiotics.
Methodology
In our prospective nonrandomized comparative study, the subjects were divided into two groups: group A treated with subcutaneous injection of unfractionated heparin plus antibiotics and group B with antibiotics alone. Comparison was done in terms of improvement in clinical parameters like erythema (cellulitis area), edema (measuring limb girth), pain (visual analog scale), total leucocyte count, and duration of hospital stay.
Results
Total of 96 patients were divided into two groups of 48 each. In the experimental group, there was improvement in mean area of cellulitis with decrease of 32.36% compared with 10.99% in controls treated with antibiotics at day 5 (p < 0.001). There was 26.27% decrease in edema compared with 12.87% (p < 0.001). There was 45.36% decrease of pain compared with 38.02% (p < 0.001). There was 29.18% drop in total leucocyte counts compared with 25.87% (p < 0.001). There was reduced duration of stay at 7.15 days compared with 9.23 days in controls.
Conclusion
The addition of heparin with antibiotic therapy in cellulitis hastens recovery with faster amelioration of symptoms and shorter hospital stay.
Keywords
cellulitis
heparin
edema
erythema
pain
Introduction
Cellulitis is an acute inflammatory condition of skin that is characterized by local pain, erythema, swelling, and local elevation of temperature.1 In developed countries the incidence of cellulitis is estimated to be approximately 16.4 to 24.6/1,000 person-years.2 Cellulitis is caused by indigenous flora colonizing the skin and appendages and associated endogenous causes.1
Most common organisms causing cellulitis are Staphylococcus aureus (51%), group A β-hemolytic Streptococcus (21%), Streptococcus pyogenes, Haemophilus influenza, and Pseudomonas aeruginosa.1 In the preantibiotics era the estimated mortality due to cellulitis was approximately 11%. Due to advent of antibiotics, this incidence was significantly reduced and only those with severe disease requiring admission suffer from mortality and overall mortality in this group of patients remains approximately 5 to 7.2%.3 Most cases of cellulitis are managed empirically. Blood culture, needle aspirate, and skin biopsies are various methods of evaluation to determine the causative organism but seldom yield significant results and are not commonly employed in majority of cases. Traditionally, cellulitis has been managed empirically with antibiotics chosen to cover staphylococci and streptococci.3 Until the antimicrobial susceptibility result is awaited, empirical treatment with aminoglycosides, third-generation cephalosporins, and semisynthetic penicillin (ticarcillin, mezlocillin, or piperacillin) is commenced until culture and sensitivity reports are available.3,4
For over 60 years heparin is being used routinely in clinical practice as an anticoagulant and antithrombotic agent. Studies have shown that the anti-inflammatory role of heparin is mainly because of the blockade of P-selectin and L-selectin.5,6 In a randomized study by Katakkar to explore the use of heparin as a topical anti-inflammatory and antithrombotic agent, the author showed that heparin application decreased the incidence of pain, redness, and swelling. They concluded that use of heparin is safe, effective, and well tolerated.7
With the background of the abovementioned studies, our study aimed at evaluating the role of heparin in the treatment of cellulitis in addition to antibiotics.
Materials and Methods
Study Place and Duration
This was a prospective comparative study conducted at two tertiary care teaching hospitals from December 2019 to August 2021.
Sample Size
From previous literature review, sample size of 96 patients was calculated to achieve significant results and study participants with cellulitis were included and randomized to two groups, group A treated with subcutaneous injection of unfractionated heparin and intravenous antibiotics and group B only with antibiotics alone.
Inclusion and Exclusion Criteria
All the patients who were diagnosed as cellulitis aged 18 years and above of both sexes were included in the study.
Patients with age less than 18 years were excluded from the study. Similarly, patients sensitive to heparin, with altered coagulation profile, history of disseminated intravascular coagulation, with peptic ulcer disease, bleeding disorders, decompensated liver disease, renal failure, intracranial bleed, and patients with sepsis with hypotension were excluded from the study. Heparin sensitivity test was done for all patients before injecting.
Study Protocol
Informed consent was taken from all patients. All the patients were subjected to clinical evaluation in the form of detailed history and physical examination.
Patients clinically diagnosed with cellulitis were randomized into two groups:
Group A (experimental)–Patients diagnosed as cellulitis treated with subcutaneous unfractionated heparin injection and intravenous antibiotics.
Group B (controls)–Patients diagnosed as cellulitis treated with only intravenous antibiotics.
Group A cases were treated with subcutaneous unfractionated heparin injection (from different manufacturers–Beparine [Biological Evans Ltd.], Bioclot [Elfin Drugs Pvt. Ltd.], and Beprin [Taj Pharmaceuticals]) of an initial dose of 5,000 units eighth hourly till swelling subsided clinically. Activated partial thromboplastin time was monitored before starting, and on 2nd and 3rd days postadministration of heparin. Intravenous antibiotics were started according to the Infectious Diseases Society of America (IDSA) guidelines in both groups.8
For group B: Only intravenous antibiotics were started according to the IDSA guidelines.
Cultures and sensitivity from any source were not done for any of the patients and they were only treated with empirical antibiotics in both groups.
Outcome Assessment
Groups A and B were compared in terms of the following outcomes:
Primary outcomes: Improvement in signs and symptoms like:
Erythema (surface area was measured by imprint of plastic sheet over graph paper and recorded in centimeter square).
Edema of limb (measuring limb girth).
Pain (by visual analog scale).
Total leucocyte counts assessed on day 1, 5, and 7 (measured by automated coulter counter).
Secondary outcomes:
Duration of stay in hospital: Patients were discharged after resolution of pain, no fever episode for duration of 48 hours, and clinical reduction in edema.
Adverse effects of infusion of heparin like bleeding, subcutaneous hematomas, or aggravation of cellulitis.
Statistical Analysis
Data was collected using standardized patient pro forma and entered in EpiData version 3.1 (JM Lauritsen. Epidata Association. Odense, Denmark, 2005) and was analyzed using SPSS version 13 (IBM Corp. Released 2009. IBM SPSS Statistics for Windows, Version 13.0. Armonk, NY: IBM Corp). Qualitative data was expressed as frequency and percentage. Quantitative data was expressed as mean (±standard deviation) and interferential analysis was performed by chi-square test, Fisher's exact test, and Student's paired t-test. p-Value of 0.05 was considered statistically significant.
Ethical Committee Approval
Scientific committee and Ethical committee approval (IEC 12 – 13/274) was obtained from the institutional ethics committee.
Results
All patients presenting with clinical diagnosis of cellulitis and willing to participate in the study were included till the sample size was reached and recruitment was stopped at completion of sample size. A total of 104 patients were screened and 8 of them refused to consent for study and 96 patients were included in the final analysis. Of the 96, 71 (74%) patients were male and 25 (26%) patients were female.
Ninety-six patients were divided into two groups of 48 each; there were no significant differences between the two groups regarding outcome-related factors at admission (day 1) as indicated in ►Table 1. Group A patients with cellulitis were treated with antibiotics and heparin, while group B patients with cellulitis were treated with antibiotics alone.
| Day 1, mean | p-Value | Day 5 | p-Value | Day 7 | p-Value | ||
|---|---|---|---|---|---|---|---|
| Group A (N) Group B (N) | 48 48 | 48 48 | 35 43 | ||||
| Mean area of cellulitis (cm2) | Group A Group B | 258.1 263.5 | 0.53 | 174.6 234.5 | < 0.001 | 160.9 217.3 | < 0.001 |
| Mean limb girth (cm) - edema | Group A Group B | 29.1 28.2 | 0.18 | 21.5 24.5 | < 0.001 | 20.7 22.1 | 0.02 |
| Mean pain score (VAS) | Group A Group B | 7.8 8.4 | 0.083 | 4.3 5.2 | 0.005 | 2.4 2.6 | 0.665 |
| Mean total leucocyte count (cells/cu.mm) | Group A Group B | 12131.7 12958.1 | 0.19 | 8591.9 9605.3 | 0.009 | 8370.5 8384.8 | 0.96 |
Abbreviation: VAS, visual analog scale.
In group A treated with both antibiotics and subcutaneous injection of unfractionated heparin, there was improvement in area of cellulitis as measured on day 5 with mean area of 174.563 cm2 (32.36% decrease of area of cellulitis) as compared with 234.5 cm2 (10.99% decrease in area) in controls treated with antibiotics alone (►Table 1; ►Fig. 1).
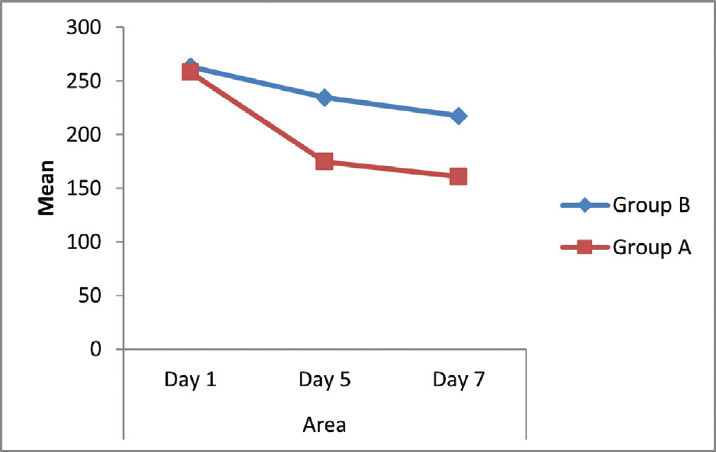
- Comparison of area of involvement of cellulitis (cm2) between the two groups.
On comparing the two groups based on reduction in limb swelling, that is, edema on day 5, there was reduction of 7.6 cm (26.3%) in the experimental group as compared with 3.7 cm (12.9%) in the control group, which was found to be statistically significant (►Table 1; ►Fig. 2).

- Comparison of limb girth (cm) between the two groups.
The mean pain score based on visual analog scale was found to be significantly lower at 4.3 points (45.36% reduction from admission) on day 5 in the experimental group as compared with 5.2 points (38.02% reduction since admission) in controls (►Table 1; ►Fig. 3).
![Comparison of pain (visual analog scale [VAS]) between the two groups.](/content/157/2023/9/1/img/IJRSMS-09-34-g003.png)
- Comparison of pain (visual analog scale [VAS]) between the two groups.
The drop is mean total leucocyte count on day 5 was 3,540 cells/cu.mm (29.18% drop) in the experimental group as compared with 3,353 (25.87% drop) in the control group, which was found to be statistically significant (►Table 1; ►Fig. 4).
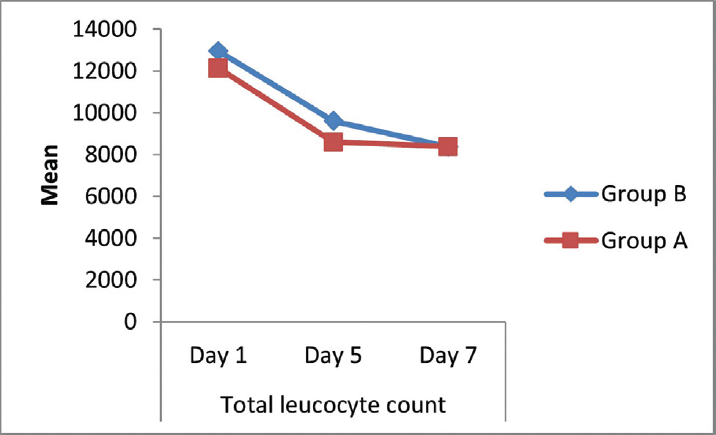
- Comparison of total leucocyte count (cells/cu.mm) between the two groups.
Mean duration of hospital stay in group A was significantly less at 7.15 (±1.2) days while group B was 9.23 (±1.5) days (p < 0.001).
There were no significant adverse events, allergic reactions, anaphylaxis, local, or bleeding-related complications due to heparin injections in the experimental group in our study. There were no antibiotic-related complications in both the groups. There was no mortality in both the groups.
Discussion
Cellulitis is a commonly encountered clinical problem and due to lack of specific therapies leads to prolonged duration of symptoms and suffering. Various guidelines for management exist and our study aimed at outcomes with use of heparin in addition to antibiotics for faster recovery and all endpoints were directed toward this. Patients in both groups were compared in terms of decrease in erythema, edema (limb girth) pain, fever, total leucocyte count on day 1, day 5, and day 7, and duration of hospital stay.
Katakkar has previously validated the use of heparin as a topical anti-inflammatory and antithrombotic agent. This randomized study showed that heparin application decreased the incidence of pain, redness, and swelling and concluded that use of heparin is safe, effective, and well tolerated.7 In our study, there was faster resolution of area of erythema, tissue edema, and pain at day 5 of treatment with heparin as compared with the control group.
Chen et al investigated the effect of heparin in second degree burn wounds in 100 patients selected randomly and concluded that the addition of heparin results in shorter healing time with less duration of stay and reduction in pain.9 In our study, patients with burns were not included but only those with cellulitis were included. However, the mechanism of inflammation in both cases is similar and anti-inflammatory effect of heparin should make it safe for use even in burn cases if there is no evidence of coagulopathy.
Jordan and Piotrowski reported usage of light density heparin in treatment of orbital phlegmonous cellulitis and thrombophlebitis of cavernous sinus and hypothesized it was responsible for stopping the process of thrombophlebitis of orbital veins and cavernous sinus thrombosis owing to its anticoagulant as well as anti-inflammatory properties.10 Heparin containing irrigation fluid has also been shown to reduce postoperative inflammation among patients undergoing cataract surgery.11
Potent anti-inflammatory property of heparin results primarily from blockade of P-selectin and L-selectin.12,13 Ability of heparin to maintain blood flow by preventing sludging and clots, anti-inflammatory effect, neoangiogenic effects that revascularizes tissues, and reduced translocation bacteria are responsible for improvement in clinical parameters.14–16 The anti-inflammatory effect of nebulized heparin has also been used to treat acute respiratory distress syndrome and reduce alveolar damage. The proposed mechanisms have been reduction in fibrin deposition and reduction in microvascular thrombosis.17,18
With the analysis of the available literature, our present study comparing the clinical outcomes suggests that there was significant decrease in area of cellulitis (erythema), edema (limb girth), pain, and total leucocyte count on day 5 in the experimental group treated with subcutaneous injection of heparin and conventional intravenous antibiotics when compared with the control group treated with conventional intravenous antibiotics alone without heparin. Duration of hospital stay was less in group A compared with group B. As the duration of stay is significantly decreased, it is found to be cost effective, and we suggest its use in regular practice.
Limitations of this study include the relatively smaller number of cases for comparison and cost of heparin has been not compared. As the duration of stay is less in cases compared with controls, it may be cost effective but overall treatment cost is not calculated to comment on the same. Patients with severe cellulitis with systemic symptoms like renal failure, hypotension, sepsis, and multiorgan dysfunction syndrome were not included so the role of addition of heparin in such cases cannot be commented. Future studies can be focused on such cases to have a broader clinical spectrum for use of heparin.
Conclusion
The authors conclude that the addition of subcutaneous injection of unfractionated heparin to conventional antibiotics in cellulitis is more beneficial when compared with the conventional antibiotics alone and lead to rapid resolution of clinical symptoms and expedited recovery with shorter duration of hospital stay. So, it may be used as a fundamental tool in the treatment of cellulitis.
Acknowledgments
None.
Conflict of Interest
None.
References
- Harrisons Principles of Internal Medicine. Vol 1. (18th). New York: McGraw Hill; 2013. p. :1067-1069.
- [Google Scholar]
- Risk stratification and outcome of cellulitis admitted to hospital. J Infect. 2010;60(06):431-439.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice. Cellulitis. N Engl J Med. 2004;350(09):904-912.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(02):295-297.
- [CrossRef] [PubMed] [Google Scholar]
- Non-anticoagulant effects of heparin: an overview. Handb Exp Pharmacol. 2012;207(207):281-305.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of surface-bound and intravenously administered heparin on cell-surface interactions: inflammation and coagulation. Perfusion. 2013;28(03):263-271.
- [CrossRef] [PubMed] [Google Scholar]
- Use of heparin as a topical antithrombotic and anti-inflammatory agent. J Natl Cancer Inst. 1993;85:1865-1866.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(03):285-292.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Topical Heparin on Second Degree Burn Wounds. 6th International Symposium Proceedings; 5th Asian Pacific Burn Congress.
- The usage of light density heparin (fraxiparin) in the treatment of orbital phlegmonous cellulitis with orbital veins and thrombophlebitis of the cavernous sinus [in Polish] Otolaryngol Pol. 1995;49(06):532-542.
- [Google Scholar]
- The effect of enoxaparin-containing irrigation fluid used during cataract surgery on postoperative inflammation in patients with diabetes. Am J Ophthalmol. 2013;156(06):1120-1124.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Old and new applications of non-anticoagulant heparin. Int J Cardiol. 2016;212(01):S14-S21.
- [CrossRef] [PubMed] [Google Scholar]
- Heparin inhibits proinflammatory and promotes anti-inflammatory macrophage polarization under hyperglycemic stress. J Biol Chem. 2020;295(15):4849-4857.
- [CrossRef] [PubMed] [Google Scholar]
- The role of heparin in sepsis: much more than just an anticoagulant. Br J Haematol. 2017;179:389-398.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated Intravascular Coagulation: An Update on Pathogenesis, Diagnosis, and Therapeutic Strategies. Clinical and Applied Thrombosis/Hemostasis. 2018;24:8S-28S.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: review on latest available drugs and therapies against SARS-CoV-2. Coagulation and inflammation cross-talking Virus Res. 2020;286:198070.
- [CrossRef] [PubMed] [Google Scholar]
- Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(04):360-372.
- [CrossRef] [PubMed] [Google Scholar]
- Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: a pilot study. Pulm Pharmacol Ther. 2018;48:88-96.
- [CrossRef] [PubMed] [Google Scholar]







