Translate this page into:
Impact of Different Histopathological Factors on Recurrence and Survival in Operated Carcinoma Esophagus
*Corresponding author: Vishal Kewlani, MBBS, DNB, PDCF, Surgical Oncology, Motilal Nehru Medical College, Allahabad, Uttar Pradesh, India. abstractvishal@gmail.com
Abstract
Background
Even with radical surgery, a significant percentage of patients of esophageal cancer experience recurrent disease.
Aims
The aim of the current study is to define the impact of different histopathological factors on the recurrence and survival in carcinoma esophagus following surgery.
Materials and Methods
A retrospective review of 182 patients of esophageal carcinoma, operated between January 2011 and December 2016, was done. In our study, 92 patients underwent upfront surgery and 90 took neo-adjuvant/perioperative treatment before planned surgery. To compare the proportion between two groups, chi-square test was used and to compare the median between the two groups, Mann—Whitney U test was used. Factors affecting the survival were analyzed using the Kaplan–Meier survival curve to compare the median survival time across groups log rank (Mantel–Cox) test was used.
Results
Out of 182 patients, 55 patients developed recurrences, in which 19 were loco-regional and 36 were systemic. Patients with lymph node-positive disease on final histopathology had more recurrence than lymph node-negative (39.74%, 31/78) versus (23%, 24/104), p = 0.01 (significant). Patients with features such as PNI-positive, poor differentiation, lymph node-positive, ENE, and higher stage disease had statistically significant, lower DFS and OS with p-value < 0.05. Patients with adenocarcinoma histology had more systemic recurrences and statistically significant lower DFS than SCC with p-value < 0.05.
Conclusions
Systemic recurrences are more common. PNI, ENE, grade, lymph node-positive disease, and higher pathologic stage had statistically significant negative impact on both DFS and OS. On multivariate analysis, whereas ENE had an impact on DFS alone.
Keywords
Esophageal carcinoma
Squamous cell carcinoma
Adenocarcinoma
Recurrence
Prognostic factor
Introduction
Even with the recent advances in the diagnosis and treatment, esophageal cancer still has significantly high mortality. The mean survival for squamous cell carcinoma (SCC) is 13.95 ± SD 11.2 months and for esophageal adenocarcinoma (EA) is 13.22 ± SD 10.23 months.[1–3] Prognostic factors linked to the patient and with the disease are numerous and not well investigated. Defining these factors will help in a better classification of high-risk groups.[4,5]
Esophageal cancer is a frequent cancer world over, leading to more than 400,000 deaths every year.[6] In the treatment protocols of esophageal carcinoma in early stage, surgical resection is commonly recommended. Nevertheless, the overall 5-year survival rate after esophagectomy remains ~25%.[7] Such unfavorable results are due to the high rate of recurrences following resection of the primary tumor. Loco-regional recurrences and distant organ metastases are identified in 50% of patients, usually in 2 to 3 years post-surgery.[8–11] Current advances in adjuvant therapy techniques may specifically benefit patients with a high risk of recurrence after surgical resection. In fact, recurrent disease sometimes has superior response to chemotherapy and radiation treatment, and those patients can achieve relatively longer overall survival. Despite radical surgery for esophageal carcinoma, a considerable number of patients develop recurrence. The objective of our current study was to evaluate the impact of diverse histopathological factors as a guide to identify recurrence and survival in postoperative patients of carcinoma esophagus.
Materials and Methods
This retrospective study was conducted at the Gujarat Cancer and Research Institute, Ahmedabad, India. The study was officially approved by the Ethics Committee of the Institute, and 182 patients treated with surgery between January 2011 and December 2016 were enrolled. All tumors were confirmed, as either squamous cell carcinoma (SCC) or adenocarcinoma by endoscopy and guided biopsy.
Inclusion criteria
Esophageal Carcinoma – Operable squamous and adenocarcinoma histology with R0 resection.
Exclusion criteria
Inoperable disease on exploration.
Histology other than squamous and adenocarcinoma.
Incomplete follow-up data.
Patients with R+ resection.
Preoperative evaluation included a thorough physical examination, an upper GI endoscopy with biopsy, CT/MRI chest, and upper abdomen to stage the disease. PET CT scan was done only in cases with bulky lymphadenopathy (>1 cm) to rule out distant metastases. All patients were discussed in the multidisciplinary tumor board before starting the treatment. As per the hospital protocol, patients with clinic-radiological T1, T2, T3, and nonbulky regional lymph node underwent upfront surgery; those with clinical T4a and bulky regional lymph nodes were planned for neoadjuvant treatment followed by surgery. In our study, 92 patients underwent upfront surgery and 90 took neo-adjuvant/peri-operative treatment before planned surgery. These included 37 patients with SCC mid and lower thoracic esophagus treated with neoadjuvant chemoradiation (45 gy RT + platinum-based chemotherapy), 33 patients with lower esophagus and gastroesophageal junction (GEJ) SCC treated with neo-adjuvant platinum-based chemotherapy, and 20 patients with lower esophagus and GEJ adenocarcinoma histology who were assigned to perioperative platinum-based chemotherapy. Patients with a tumor primarily located in the lower thoracic esophagus and gastro-esophageal junction underwent either Ivor–Lewis procedure with intra-thoracic anastomosis or transhiatal esophagectomy with left cervical anastomosis. Patients with a primary tumor in the middle thoracic esophagus underwent 3-stage total esophagectomy and cervical anastomosis.
Patients were regularly followed up in OPD every 3 to 4 months, for the first 2 to 3 years, and every 6 to 12 months thereafter. Follow-up was done until January 2020 or diagnosis of recurrence. Follow-up imaging included a CT scan (thorax, abdomen, and pelvis), performed at least every 6 to 12 months for the first 2 to 3 years and thereafter as clinically indicated. The diagnosis of recurrence was made by cytology and histopathology of tissue biopsy from suspected recurrence site. Loco-regional recurrence included regional lymph nodes and anastomotic site recurrence, whereas distant included non-regional lymph nodes and systemic metastasis.
Follow-up data of patients were obtained by reviewing the medical records or by directly contacting the patient or their family over the telephone. Disease-free survival was defined as the time from the date of the surgery to the first proven recurrence. Overall survival was calculated as the time between diagnosis and death or last follow-up.
Clinical data regarding cancer histology, disease stage, tumor grade, lymph node status, lympho-vascular invasion (LVI), perineural invasion (PNI), extra nodal extension (ENE) and recurrence after surgery were collected.
Statistical analysis
All calculations were done with Statistical Product and Service Solutions (SPSS, version 17) to compare the median survival time across groups, log rank (Mantel–Cox) test was used, and p-value less than 0.05 was considered statistically significant. To compare the proportion between two groups chi-square test was employed and compare median between two groups Mann–Whitney U test was used. Factors affecting the survival were analyzed using the Kaplan–Meier survival curve.
Results
Among these 182 patients included in the study, only 74 (40.66%) patients were alive at the time of final analysis; the rest 108 (59.33%) died of various causes. The most common cause of death was disease recurrence seen in 51 patients, followed by death due to complications related to cardio-pulmonary systems. The cause of death was not known in 13 patients. Patient’s demographic and histopathological features are enumerated in Table 1.
No. of patients |
182 |
|
|---|---|---|
Sex |
Male |
96 |
Female |
86 |
|
Median age |
Male |
53 y |
Female |
50 y |
|
Histology |
Squamous Cell Carcinoma |
139 |
Adenocarcinoma |
43 |
|
Pathological stage |
PCR |
13 |
Stage I |
33 |
|
Stage II |
73 |
|
Stage III |
63 |
|
Lymph node |
Positive |
78 |
Negative |
104 |
|
Recurrence |
Loco-regional |
19 |
LVI (+)ve |
44 |
|
PNI (+)ve |
14 |
|
ENE (+)ve |
24 |
|
Pattern of recurrence
Of the 74 patients who were alive, 4 were having recurrent disease. Thus, 30.22% (55 of 182) patients following esophageal resection developed recurrent disease. The pattern of recurrence was locoregional in 19 (10.44%) and distant in 36 (19.78%) patients, respectively. The overall median time to recurrence was 12.2 months (range, 1.5–36 months). In patients with loco-regional recurrence, the median time to recurrence was 12.8 months (range, 2–29 months) and for distant metastases, it was 11.6 months (range, 1.5–36 months).
Impact of histology, pathological stage, and lymph node positivity on systemic and locoregional recurrence
Adenocarcinoma had statistically significantly more systemic recurrence than SCC, 34.88% (15/43) vs. 15.11% (21/139), (p = 0.006). However, there was no correlation for loco-regional recurrence pattern in two different types of histology (p = 0.78). Out of a total of 55 patients who developed recurrence, 4/33 (12.12%) patients with stage-I, 26/73 (35.62%) in stage-II, and 25/63 (39.68%) in stage-III developed recurrence (p = 0.028). Patients with lymph node-positive disease on final histopathology had more recurrence than node-negative 31/78 (39.74%) versus 24/104 (23%) patients, p = 0.01 was significant.
Impact of neoadjuvant/preoperative treatment on recurrences and survival
Among 92 patients treated with upfront surgery, 33 (35.87%) had recurrent disease (3 at local anastomotic site, 10 in regional lymph nodes, and 20 distant sites). Among 90 patients treated with combined modality (neoadjuvant chemoradiation/NACT/perioperative chemotherapy followed by surgery), 22 (24.44%) developed recurrences (one at the local anastomotic site, 5 in regional lymph nodes and 16 distant sites). Thirteen patients had PCR (pathological complete response) following neoadjuvant treatment and none of these patients having a PCR-developed recurrence. Though patients treated with combined modalities had less recurrences, but it was not statistically significant (p = 0.09).
The median OS in upfront surgery and combined modality group was 16.25 and 19.5 month respectively, which was statistically nonsignificant (p = 0.7).
The median DFS in the upfront surgery group and combined modality group was 12 and 12.5 month respectively, which was statistically nonsignificant (p = 0.71).
Impact of different prognostic factors on DFS and OS
The impact of multiple prognostic factors on DFS and OS was calculated using the Kaplan–Meier survival curve [Figures 1–3]. As the minimal follow-up period in our study was 12 months, only 1-year DFS and OS was calculated. Patients with PNI-positive disease had statistically significant lower DFS and OS than PNI-negative disease with a p-value < 0.05. Patients with adenocarcinoma histology had statistically significant lower DFS than SCC with a p-value < 0.05, but the impact of histology on OS was non-significant with a p-value > 0.05. Patients with poorly differentiated tumor had statistically significant lower DFS and OS than moderately to well-differentiated tumor with a p-value < 0.05. Patients with lymph node-positive disease on final histopathology had statistically significant lower DFS and OS than node-negative disease with a p-value < 0.05. Patients with stage III disease on final histopathology had statistically significant lower DFS and OS with a p-value < 0.05. LVI had a statistically nonsignificant impact on both DFS and OS with a p-value > 0.05. Patients with the ENE-positive disease had lower 1-year DFS and OS than ENE-negative disease with a p-value of < 0.05, which was statistically significant [Table 2].
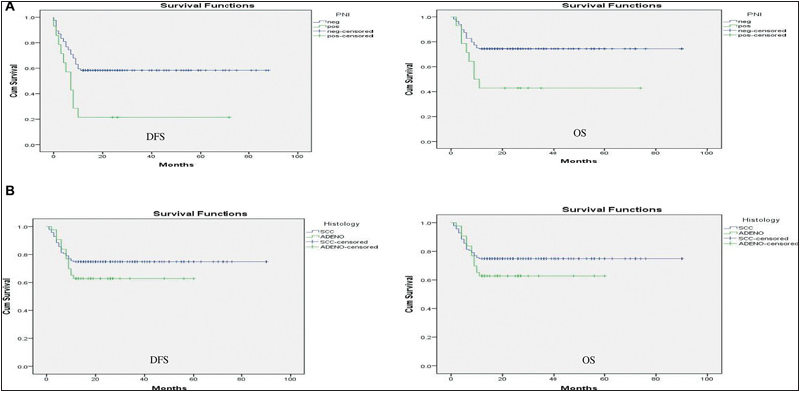
- (A) Impact of PNI on DFS and OS. (B) Impact of histology on DFS and OS.
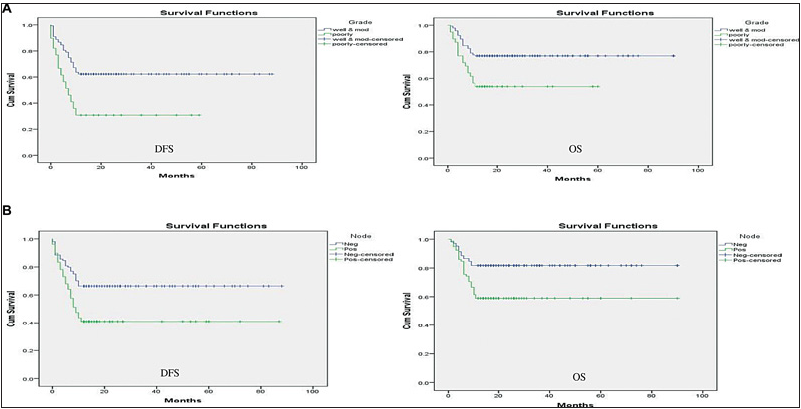
- (A) Impact of grade of differentiation on DFS and OS. (B) Impact of node-positive status on DFS and OS.
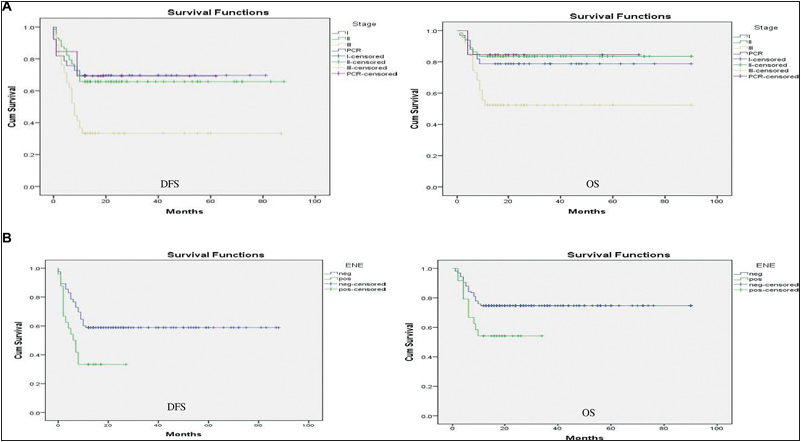
- (A) Impact of histopathological stage on DFS and OS. (B) Impact of ENE on DFS and OS.
Variables |
Impact on DFS |
Impact on OS |
||||
|---|---|---|---|---|---|---|
Chi square test |
p-value |
Significance |
Chi-square test |
p-value |
Significance |
|
LVI |
0.74 |
0.38 |
No |
1.55 |
0.21 |
No |
PNI |
8.93 |
0.003 |
Yes |
6.5 |
0.01 |
Yes |
ENE |
9.02 |
0.003 |
Yes |
4.81 |
0.028 |
Yes |
Histology |
6.22 |
0.013 |
Yes |
1.64 |
0.2 |
No |
Grade |
16.72 |
0.000 |
Yes |
9.09 |
0.003 |
Yes |
Node (+)ve |
11.47 |
0.001 |
Yes |
10.51 |
0.001 |
Yes |
Stage |
19.48 |
0.000 |
Yes |
17.0 |
0.001 |
Yes |
Multivariate analysis
On multivariate analysis, lymph node-positive status, higher stage, poor differentiation, and PNI had statistically significant poor 1-year DFS and OS. ENE had statistical significance on DFS alone. LVI had no statistically significant impact on DFS and OS. In the above multivariate analysis, the histology of the tumor was kept as a constant parameter and analysis was performed for other histopathological variables [Table 2].
Discussion
Radical surgery in form of esophagectomy with radical lymph node dissection is the principal modality in the treatment for esophageal cancer. Yet, the rich lymphatic capillary network in the esophageal mucosa and submucosa leads to a high incidence of local recurrences or distant metastases after surgery. It has been identified that within 2 to 3 years after the surgery, more than half of esophageal cancer patients develop recurrence or metastases.[8,9] Mariette et al[10] have reported that 90% of recurrence occur within 38 months after surgery.
Neoadjuvant chemoradiotherapy has been found to improve local regional control and extend the survival in patients with locally advanced esophageal cancer. Recurrence at locoregional sites is still reported in 13 to 22% of patients considered to have a pathological complete response following neoadjuvant therapy.[12,13] In the present study, 35.87% (33/92) developed recurrences in the upfront surgery settings, whereas 24.44% (22/90) developed in the combined modality group, which is similar to the findings in other studies.
Systemic recurrences were found to have adverse impact on prognosis. with most frequent sites of hematogenous metastases being liver, lungs, and bones.[8,10,14] In Su et al’s study, the most common sites for distant recurrence were still the lungs, liver, and bones, accounting for 81.8% of all metastases.[15] The present study also confirmed the above findings with the liver, lungs, and bones accounting for greater than 58.90% (21/36) of distant recurrences.
Out of 55 patients who developed recurrences, 16 took some form of treatment for recurrence in the form of chemotherapy alone, radiotherapy alone, and chemo-radiotherapy. In our study, none of these patients were taken for metastasectomy. Post recurrence average survival in these patients who took treatment was 7.25 months versus 5.95 months for those who did not take treatment for recurrences.
Schoppmann et al have reported that “both the 5-year OS (14% vs. 60%, p < 0.001) and the 5-year DFS (14% vs. 49%, p < 0.001) were significantly reduced in patients with positive LVI.”[16] Chen et al. have also reported that “SCC patients with PNI-negative tumors had a 1.7- fold increase in the 5-year recurrence-free survival over the 5-year DFS or with patients with PNI positive tumors.”[17] As for ECI, d’Annoville reported that the “proportion of ECI detected in N1, N2, and N3 patients was 28% (21 of 73 patients), 51% (21 of 41 patients), and 70% (17 of 24 patients), respectively. The presence of ECI seems to have a negative additive impact on DFS, regardless of the pN stage.”[18] ECI (extra capsular invasion)/ENE identified after preoperative chemoradiation has been reported to be coupled with a very poor prognosis. In D’Journo’s study, the 5-year DFS rates were 46% in N0 patients, 36% in N+ with intracapsular invasion patients, and 11%, in N+ with ECI/ENE patients.[19]
In the present study, SCC histology was associated with superior 1-year % DFS and OS compared with adenocarcinoma histology, i.e., 55.59 (48.82–62.35 with 95% CI) versus 25.65 (17.94–33.36 with 95% CI) for DFS and 68.64 (62.52- 74.77 with 95% CI) vs. 40.32 (32.67–47.97 with 95% CI) for OS. Similarly, nodal positivity was associated with inferior 1-year DFS and OS compared with node-negative disease, i.e., 38.70% (29.75–47.66 with 95% CI) vs. 60.03% (52.47–67.58 with 95% CI) for DFS and 55.64% (46.49–64.79 with 95% CI) vs. 74.48% (68.16–80.79 with 95% CI) for OS [Table 3].
Variables |
Impact on DFS |
Impact on OS |
||||
|---|---|---|---|---|---|---|
Chi-square test |
p-value |
Significance |
Chi-square test |
p-value |
Significance |
|
LVI |
0.419 |
0.51 |
No |
1.0 |
0.3 |
No |
PNI |
7.60 |
0.006 |
Yes |
5.99 |
0.014 |
Yes |
ENE |
6.51 |
0.01 |
Yes |
3.29 |
0.069 |
No |
Grade |
11.98 |
0.001 |
Yes |
7.05 |
0.008 |
Yes |
Node + |
9.1 |
0.003 |
Yes |
9.01 |
0.003 |
Yes |
Stage |
15.86 |
0.001 |
Yes |
14.87 |
0.002 |
Yes |
Patients with poorly differentiated histology (Grade III) had inferior 1-year DFS and OS compared with moderately to well-differentiated histology, i.e., 21.15% (13.19–29.17 with 95% CI) vs. 56.79% (50.21–63.36 with 95% CI) and 34.87% (26.32–43.41 with 95% CI) vs. 70.60% (64.79–76.41 with 95% CI). LVI-positive disease on histology was associated with inferior 1-year DFS and OS compared with LVI-negative disease, i.e., 38.52% (28.61–48.43 with 95% CI) vs. 52.51%(45.65–59.37 with 95% CI) for DFS and 49.52% (39.94–59.10 with 95% CI) vs. 68.54% (62.39–74.68 with 95% CI) for OS [Table 3].
PNI-negative disease had a superior 1-year DFS and OS compared with PNI-positive disease. Corresponding survival values were 53.42% (47.22–59.61 with 95% CI) vs. 19.35% (4.88–33.83 with 95% CI) for DFS and 68.41% (62.84–73.98 with 95% CI) vs. 35.42% (17.89–52.96 with 95% CI) for OS. ENE-negative disease had superior 1-year DFS and OS compared with ENE-positive disease, i.e., 53.98% (47.63–60.34 with 95% CI) vs. 11.50% (7.03–15.06 with 95% CI) for DFS and 68.70% (62.99–74.47 with 95% CI) vs. 20.95% (15.24–26.67 with 95% CI) for OS [Table 3].
On multivariate analysis, lymph node-positive status, higher stage, poor differentiation, and PNI had statistically significant poor 1-year DFS and OS. ENE was found to have a statistical significance on DFS alone. LVI had no statistically significant impact on DFS and OS. In the multivariate analysis, the histology of the tumor was kept as a constant parameter and analysis was performed for other histopathological variables.
There were several limitations in our analysis, including retrospective nature, the impact of various treatment regimes not analyzed separately, only 1-year DFS and OS could be calculated to shorter duration of follow-up. Adjuvant treatment protocols in this study used dissimilar doses and schedules, so optimal treatment regimen could not be established.
Conclusion
Our study documented that recurrence is frequent following surgical management of carcinoma esophagus. More common are the systemic recurrences in comparison to locoregional recurrence. Adenocarcinoma, particularly has a significantly higher rate of systemic recurrence in comparison to squamous cell carcinoma. PNI, ENE, grade, nodal positivity, and final stage had statistically significant negative impact on both DFS and OS, whereas histology had a significant effect on OS alone, LVI had no impact on DFS and OS.
Conflict of interest
None declared.
References
- Metastatic lymph node ratio, 6th or 7th AJCC edition: witch is the best lymph node classification for esophageal cancer? Prognosis factor analysis in 487 patients. Arq Bras Cir Dig. 2015;28:94-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Esophageal carcinoma is squamous cell carcinoma different disease compared to adenocarcinoma? a transversal study in a quaternary high volume hospital in Brazil. Arq Gastroenterol. 2016;53:44-8.
- [CrossRef] [PubMed] [Google Scholar]
- Standardized clinical pathways for esophagectomy are not a reality in Brazil, even with a high prevalence of esophageal cancer and achalasia. Arq Bras Cir Dig. 2015;28:190-2.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of urgent esophagectomy in esophageal perforation. Arq Bras Cir Dig. 2014;27:247-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of minimally invasive surgery in the treatment of esophageal cancer. Arq Bras Cir Dig. 2014;27:237-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545-53.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg. 2004;198:205-11.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg. 2007;31:1107-14.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616-23.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors that influence early death due to cancer recurrence after extended radical esophagectomy with three-field lymph node dissection. Ann Surg Oncol. 2011;18:2961-7.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg. 2013;100:267-73.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138:1309-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg. 2011;15:558-65.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors in patients with recurrence after complete resection of esophageal squamous cell carcinoma. J Thorac Dis. 2014;6:949-57.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery. 2013;153:526-34.
- [CrossRef] [PubMed] [Google Scholar]
- The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:313.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic impact of the extracapsular lymph node involvement on disease-free survival according to the 7th edition of American Joint Committee on Cancer Staging System. Eur J Cardiothorac Surg. 2013;44:e207-e11. discussion e211
- [CrossRef] [PubMed] [Google Scholar]
- Extracapsular lymph node involvement is a negative prognostic factor after neoadjuvant chemoradiotherapy in locally advanced esophageal cancer. J Thorac Oncol. 2009;4:534-9.
- [CrossRef] [PubMed] [Google Scholar]








