Translate this page into:
The Percutaneous Endoscopic Lumbar Interbody Fusion (PELIF): An Advanced and Innovation Technique
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The degenerative spine changes, and its costs, have increased with high rates of work absenteeism and difficult clinical management. The search for minimally invasive treatments, with better results and early patients recover, with rapid hospital discharge are alternatives for these problems. The percutaneous endoscopic lumbar interbody fusion (PELIF) is a new and advanced option.
Keywords
endoscopic
fusion
spine
Introduction
Lumbar pain is an important cause of incapacitation for work, reaching at 90% of the population,1 with an alarming rate of absenteeism.2 Its treatment is difficult with ineffective responses to the clinical treatment.3 Modern surgical alternatives have provided better results and healing potential.4 The search for alternative treatments, with lower cost and faster patients'return to their work activities, has increased too.5 The authors report an advanced and innovative technique of endoscopic fusion in response to these problems.
Case Report
The patient is a 36-year–old male with left lombociatalgia for 2 years. He was treated with physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), and bed rest without any improvement. Physical examination showed Laségue's signs on the left foot, positive Valsalva's maneuver, dorsiflexion grade IV, and hypoesthesia in the anterior aspect of the left foot. The magnetic resonance imaging (MRI) showed extruded left foraminal disc herniation at L4 and L5, associated with segmental instability at L3 and L4 and L4 and L5, as a result of interspinal lesions and facet joint subdislocation. The patient, with no clinical improvement after 2 years of nonsurgical treatment, agreed with surgical treatment by foraminal disc decompression at L4 and L5 and percutaneous endoscopic lumbar interbody fusion (PELIF) at L3 and L5.
Surgical Technique
The computed tomography and MRI showed the interest area and the entry point distance to be puncture. This avoids unnecessary resections or neural tissue manipulations (Fig. 1).

- The computed tomography and magnetic resonance imaging show the entry point distance to be puncture.
With the use of the image enhancer, the puncture was performed with an 18G needle in the patient under general anesthesia. The intervertebral disc was accessed through the transforaminal route, followed by discography with iodinated contrast and methylene blue. A guide wire was positioned through the needle, sliding it to the center of the disc (Fig. 2). Successive dilators were placed on the guidewire and a beveled working cannula (7.1 mm of inner diameter) was placed on the dilators, which were then removed (Fig. 3), and the endoscope (6.9 mm of outer diameter, 6.1 mm of working channel, and 20-degree view angle) was inserted. The endoscope permits direct discal lesion visualization and the surrounding neural structures; It approaches and ressects just the necessary, without neural manipulations and avoiding instability or postoperative fibrosis.

- Discography of the disc space and placement of guide wire.
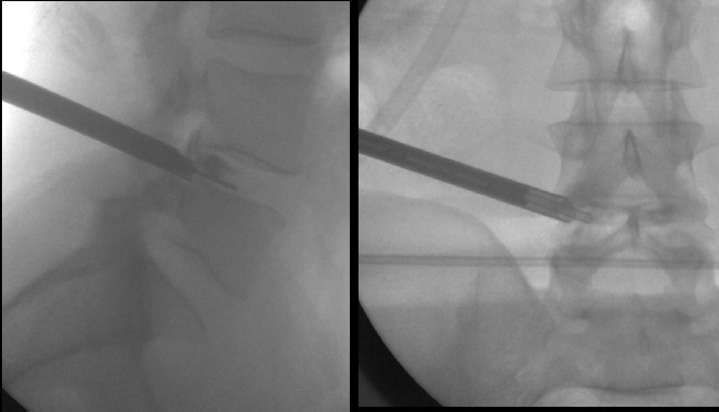
- Successive dilators are placed on the guide wire and the endoscope is inserted.
Initially, the surgeon conducted the endoscopic visualization to the facet's superior articular process (SAP), with endoscopic partial facetectomy, using an endoscopic drill system6(Fig. 4). This enabled a decompression of the neural foramen, under direct visualization of neural structures, and provided us the sufficient space for more safe and simple surgical procedures. So, a new guidewire is then inserted through the working channel of the endoscope, being positioned in the center of the disc space. The endoscope was then withdrawn, with the certainty that no neural structure was interposed or close to the working cannula, and successive tubular dilators were passed over the guide wire in a total of four, with the latter having a working channel of 15.0 mm (inner diameter; Fig. 5) that allowed the reaming disc space and endplate preparation under fluoroscopic and endoscopic guidance. After this, the polyetheretherketone (PEEK) transforaminal lumbar intersomatic fusion (TLIF) CAGEs (8–10 mm of height) was inserted (Fig. 6), without risk to the neural structures. The two CAGEs were placed under fluoroscopic and endoscopic guidance, positioned parallel, and in the anterior third of the intersomatic space for better mechanical support (Fig. 7). The endoscope is reintroduced through the 15 mm cannula for direct final visualization of the CAGES and bone graft in the intersomatic space (Fig. 8).

- The endoscopic visualization to the facet's superior articular process, with endoscopic partial facetectomy.

- The latter tubular dilators of 15.0 mm (inner diameter).

- The remaining disc space and endplate preparation.

- The two CAGEs are placed under fluoroscopic and endoscopic guidance.

- The final endoscope visualization of the CAGES in the intersomatic space.
The spinal fixation was followed by the use of a percutaneous pedicular screw system6 (Fig. 9). This appropriately sized 6 or 7 mm in diameter pedicle screws were then inserted and bilateral connecting rods were passed subfascially; following final tightening of the set screws, the rod and screw extenders were removed. After all instruments were removed, direct closure of the skin was done. No drainage was required. The patient was discharged after 2 days.

- The spinal fixation with the use of a percutaneous pedicular screw system.
Discussion
The percutaneous TLIF (pTLIF), which can be performed with an incision of only 1.5 cm, leading to a hospital discharge with only 24 hours of patient hospitalization was proposed by Morgenstern in 2010.7 He reviewed his results in 2013 and 2015,8,9 concluding that the technique has the same efficacy and safety of open lumbar arthrodesis, with a faster and more regular recovery of the patients than in the conventional method, the bone structure and the surrounding soft parts being even less aggressive.
Lee10 also showed that the advantages of the percutaneous technique are small diameter incisions, with no-bone or soft-tissue lesions, the facet joint is not resected, stability is not compromised, there is no manipulation of neural structures, fistula, or neurological impairments, bleeding is negligible and has a low risk of infection. We used the PEEK TLIF intersomatic CAGE to restore the disc height. However, Krishnan11 has showed that the cost of instrumented expandable cage may make it nonfeasible, and the percutaneous transforaminal endoscopic decompression and cageless percutaneous bonegraft, with a good endplate preparation, have greater fusion rates.
Conclusion
The treatment of degenerative disc diseases is still controversial and much has to be advanced; however, the combination of endoscopic decompression techniques, associated with percutaneous pedicle screw fixation, has shown an excellent cosmetic-functional final result (Fig. 10), with a safe and effective option to neural decompress interbody fusion. The PELIF is a new and advanced option to effective interbody fusion in full endoscopic view without technical limitations.

- The final excellent cosmetic-functional result.
Conflict of Interest
None declared.
References
- Intradiscal electrothermal annuloplasty. Am J Phys Med Rehabil. 2005;84(07):538-549.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of workers' compensation claimants with low back pain undergoing intradiscal electrothermal therapy. Spine. 2004;29(04):435-441.
- [CrossRef] [PubMed] [Google Scholar]
- Side effects and complications after percutaneous disc decompression using coblation technology. Am J Phys Med Rehabil. 2006;85(01):6-13.
- [CrossRef] [PubMed] [Google Scholar]
- Intradiscal pressure study of percutaneous disc decompression with nucleoplasty in human cadavers. Spine. 2003;28(07):661-665.
- [CrossRef] [PubMed] [Google Scholar]
- Temperatures within the lumbar disc and endplates during intradiscal electrothermal therapy: formulation of a predictive temperature map in relation to distance from the catheter. Spine. 2004;29(10):1124-1129. discussion1130–1131
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive multi-level posterior lumbar interbody fusion using a percutaneously inserted spinal fixation system: technical tips, surgical outcomes. J Korean Neurosurg Soc. 2011;50(05):441-445.
- [CrossRef] [PubMed] [Google Scholar]
- Full endoscopic transforaminal lumbar interbody fusion approach with percutaneous posterior transpedicular screw fixation in a case of spondylolisthesis grade I with L4–5 central stenosis correspondence. J Crit Spine Cases (03):115-119.
- [Google Scholar]
- Endoscopically assisted transforaminal percutaneous lumbar interbody fusion.Endoscopic Spinal Surgery. London: JP Medical Publishers; 2013. p. :129-134.
- [CrossRef] [Google Scholar]
- Percutaneous transforaminal lumbar iInterbody fusion (pTLIF) with a posterolateral approach for the treatment of denegerative disk disease: feasibility and preliminary results. Int J Spine Surg. 2015;9(09):41.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous transforaminal lumbar interbody stabilization.Minimally Invasive Percutaneous Spinal Techniques. Philadelphia, PA: Elsevier Saunders; 2011. p. :367-73.
- [CrossRef] [Google Scholar]
- Percutaneous transforaminal endoscopic decompression and cageless percutaneous bone graft transforaminal lumbar interbody fusion: a feasibility study. J Orthopaedics Allied Sciences. 2018;6(03):21-27.
- [CrossRef] [Google Scholar]







