Translate this page into:
Adalimumab-Induced Isolated Peritoneal Tuberculosis
*Corresponding author: Elias Saikaly, General Surgery Department Saint George Hospital University Medical CenterLebanon. dreliassaikaly@gmail.com
How to cite this article: Saad MK, Ghandour F, Saikaly E. Adalimumab-Induced Isolated Peritoneal Tuberculosis. Int J Recent Surg Med Sci. 2024;10:S96-100. doi: 10.1055/s-0043-1761477
Abstract
The therapeutic approach to immune-mediated diseases including Crohn’s disease has dramatically improved with the introduction of immunomodulators such as anti-tumor necrosis factor (TNF). However, its use is not complication-free, and since its introduction, a growing number of opportunistic infections is being reported in patients under treatment despite all preventive measures taken. Herein, we report a case of adalimumab-induced isolated peritoneal tuberculosis.
Keywords
Adalimumab
Extrapulmonary TB
Isolated peritoneal tuberculosis
INTRODUCTION
The US Food and Drug Administration has approved three anti-tumor necrosis factor TNF medications for the treatment of inflammatory conditions such as Crohn’s disease. The three anti-TNFs are infliximab, adalimumab, and etanercept. These agents suppress the activity of TNF, which is an essential component in the human immune response to infections. Having said this, a growing number of anti-TNF-induced tuberculosis infections is being reported since the introduction of anti-TNFs. In fact, some reports showed an increase in the risk of tuberculosis infection from 1.6-fold to 25-fold when using anti-TNFs.[1] Furthermore, it has been documented that among the three anti-TNFs, adalimumab has the highest incidence of tuberculosis infection with 144 events per 100,000 person-years, followed by infliximab with 136 events per 100,000 person-years, and finally etanercept with 39 events per 100,000 person-years.[2] The mechanism behind this infection is thought to be related to the mechanism of action of anti-TNFs by inhibiting granuloma formation, which is needed to control bacterial overgrowth and limit bacterial dissemination during tuberculosis infection. Hence, this mandates screening for tuberculosis before initiating the therapy, and tuberculosis infection should always be considered when clinically applicable in patients taking anti-TNFs. Furthermore, the absence of specific signs and symptoms that leads clinicians to diagnose such an opportunistic infection is the main challenge. Even more challenging is the diagnosis of isolated peritoneal tuberculosis in patients on anti-TNFs, especially with overlapping signs and symptoms with different pathologies including liver cirrhosis, autoimmune deficiency syndrome, and malignant conditions.
CASE REPORT
A 70-year-old male patient with a previous surgical history of open cholecystectomy 50 years ago known to have Crohn’s disease, confirmed by histology obtained from the terminal ileum by colonoscopy diagnosed 2 years before presentation, currently on adalimumab for the last 7 months, was referred to our institute for the evaluation of increased abdominal girth and night sweats. On questioning, the patient reported 25 kilograms of unintentional weight loss over a period of 2 months associated with night sweats and early satiety. The patient denied a change in bowel habits, hematochezia, melena, change in stool caliber, and change in skin color. The patient denies any chronic disease, no alcohol abuse, no family history of malignancy, and no use of herbal medications. On physical examination, vital signs were within normal limits with a body temperature of 36.8°C, 120/70 arterial blood pressure, and 72 beats/min heart rate. Abdominal examination revealed positive bowel sounds, distended abdomen, dullness to percussion in bilateral lower quadrants, no tenderness on palpation, and no rebound tenderness. Laboratory studies showed no anemia, no leukocytosis, normal electrolytes with normal renal function, and normal liver function tests. However, C-reactive protein (CRP) was 59 (normal range: 0–5 mg/L) and erythrocyte sedimentation level was 62 (normal range: 0–20 mm/h). Furthermore, a chest X-ray was done and turned out to be normal with no infiltrations or effusions. However, abdominal ultrasound showed abdominal free fluid and multiple implants in peritoneal surfaces with possible omental cake. Consequently, an ultrasound-guided paracentesis and omental biopsy were done and sent for pathology, culture, and laboratory studies. Pathology did not detect any malignant cells, and the fluid was rich in inflammatory cells. Serum albumin and ascites gradient were in favor of exudate. Tuberculosis quantiFERON PCR was negative, and ascites fluid direct microscopic examination revealed that there were no tuberculosis bacilli and the acid-fast stain was negative. Furthermore, this was followed by blood tests for tumor markers including CEA, Ca19-9, AFP, and Ca 125, which turned out to be normal. Consequently, a computed tomography (CT) scan of the abdomen and pelvis was performed showing a large amount of ascites [Figure 1] and possible omental cake [Figure 2]. At this point, the patient was scheduled for diagnostic laparoscopy and surgical biopsies from the omentum and peritoneum. Intraoperatively, a greenish ascetic fluid was noted [Figure 3] and whitish nodules on the peritoneum, omentum, and small bowel [Figure 4]. Histopathology examination revealed sections of omental fat, with numerous well-defined granulomas. Most of the granulomas showed central necrosis. A mild background lymphocytic cell infiltrate was seen. Peritoneal fat with extensive, well-defined, necrotizing granulomas was observed [Figures 5 and 6]. Figure 7 shows well-defined granulomas with central necrosis.
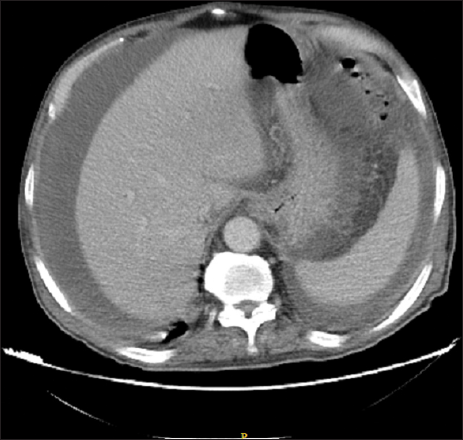
- CT scan of the abdomen showing a large amount of ascites.
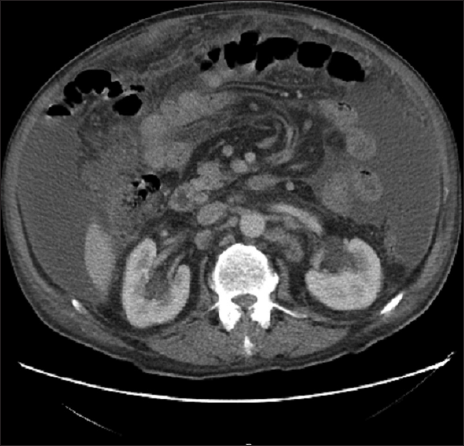
- CT scan of the abdomen showing omental cake.
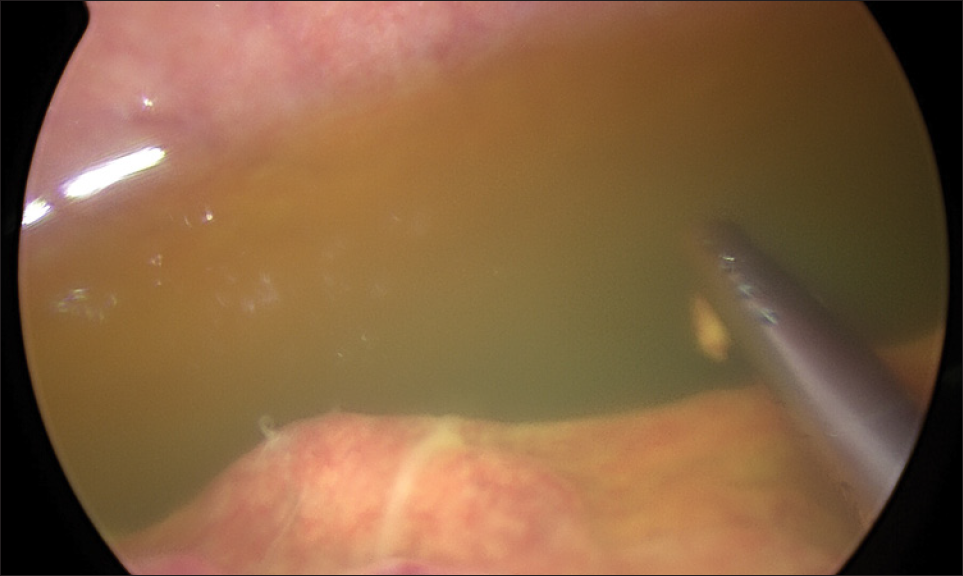
- Intraoperative image of ascetic fluid.
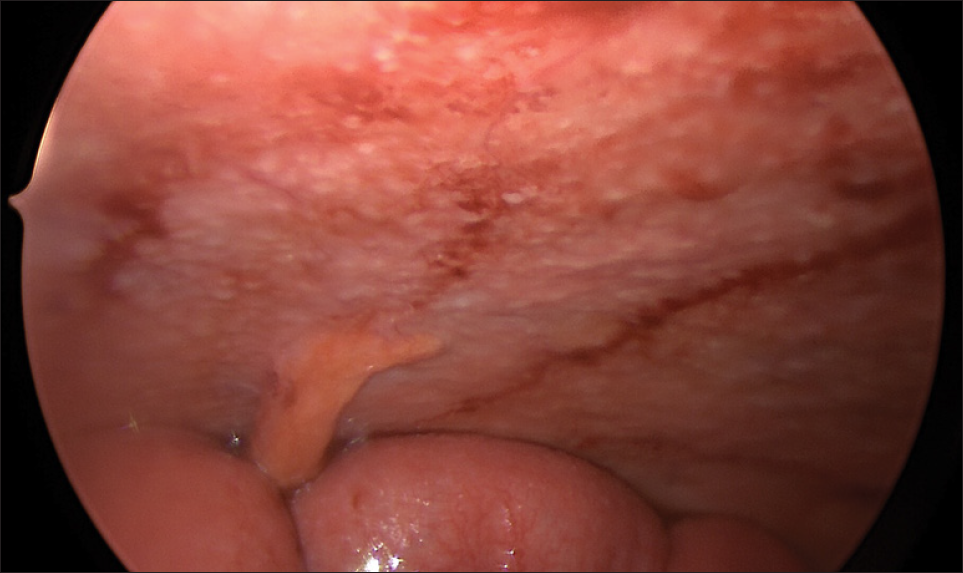
- Intraoperative image showing peritoneal nodules and thickening of the small bowel.
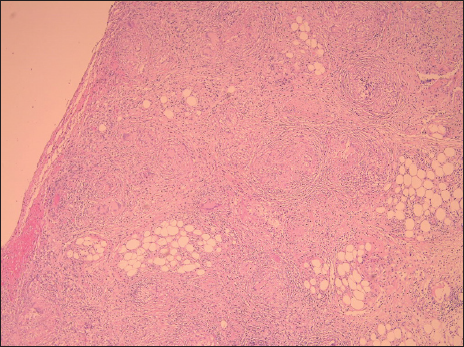
- Low-power view showing peritoneal fat with extensive, well-defined, necrotizing granulomas.
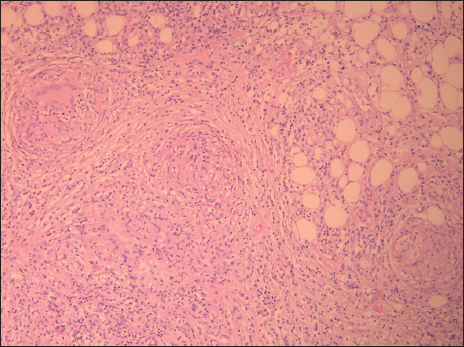
- High-power view showing peritoneal fat with extensive, well-defined, necrotizing granulomas.
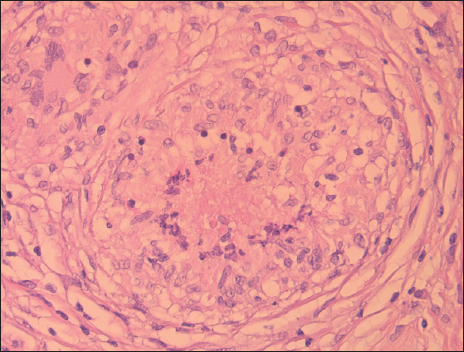
- Well-defined granuloma with central necrosis.
Afterward, the treatment was initiated for abdominal TB with the conventional antituberculous therapy for 6 months including an initial 2 months of rifampicin, isoniazid, pyrazinamide, and ethambutol, along with vitamin B6 supplements and possible extension to 12 or 18 months upon reevaluation of the patient.
DISCUSSION
The therapeutic approach to immune-mediated diseases including Crohn’s disease has dramatically improved with the introduction of immunomodulators such as anti-TNF. It is well known that TNF plays a major role in the defense against Mycobacterium tuberculosis. From here and since its introduction, a growing number of opportunistic infections is being reported in patients under treatment despite all preventive measures taken. In fact, since the approval of the first anti-TNF-α in 1998, there has been a noticeable rise in the incidence of tuberculosis infections in this patient population,[3] with a greater proportion of these cases have been extra pulmonary compared with the general population.[3] Having said this, most countries have established well-defined guidelines to screen for TB before initiating anti-TNF medications to prevent future TB during the course of treatment. However, an increasing number of reported cases of TB have been reported in patients under anti-TNF therapy. Currently, it is believed that the reactivation of latent TB is the main etiology behind this opportunistic infection in most reported cases.
In contrast, a few cases of anti-TNF-induced isolated PTB have been reported in the medical literature. PTB is difficult to diagnose and is considered a challenge to the unaware. This difficulty is mainly due to its nonspecific features, with manifestations overlapping with different conditions including liver cirrhosis, autoimmune deficiency syndrome, and malignant conditions. In addition, signs, symptoms, and laboratory findings are neither specific nor diagnostic. Presenting symptoms include abdominal pain, weight loss, anorexia, fever, diarrhea, constipation, recurrent bowel obstruction, and increasing abdominal girth. In contrast, laboratory abnormalities include anemia, leukocytosis, hypoalbuminemia, and elevated blood sedimentation rate.[4] CT-scan is the most reliable imaging technique for evaluation although no pathognomonic radiological finding is present.[5,6] Diagnosis can be confirmed with PCR on tissue biopsy, culture, and Ziehl–Neelsen staining for acid-fast bacilli.[7,8]
Surgical biopsies, either through open traditional surgery or laparoscopic surgery, have been shown to be superior to percutaneous peritoneal biopsy in obtaining the culture and specimen. Furthermore, the advantage of the laparoscopic approach is that it allows macroscopic visualization of the peritoneum, which may reveal whitish nodules scattered throughout the peritoneum along with ascites, thickened omentum, and thickened bowel loops, or yellowish nodules with adhesions.[9] Histologic evaluation reveals AFB on staining in up to 75% of cases and caseating granulomas seen in 85% to 90% of cases.[10]
Once diagnosed, anti-TNF medications should be ceased and medical treatment for TB initiated. Treatment of abdominal TB is conventional antituberculous therapy for at least 6 months including an initial 2 months of rifampicin, isoniazid, pyrazinamide, and ethambutol, with possible extension to 12 or 18 months.[11]
In our case following the abdominal CT scan, exploratory laparoscopy was scheduled as this allows for complete inspection of the peritoneal cavity and allows surgical biopsies for histologic examination.
CONCLUSION
In conclusion, the increased risk for reactivation of latent TB in patients being treated with anti-TNF is currently well established, especially in extrapulmonary locations. Isolated PTB is difficult to diagnose and is considered a challenge to the unaware. Hence, a high index of suspicion is needed for its diagnosis. Clinicians dealing with this patient population should aggressively pursue tissue biopsies for culture and histologic examination.
Conflict of interest
None declared.
References
- The Risk of Tuberculosis Related to Tumour Necrosis Factor Antagonist Therapies: A TBNET Consensus Statement. Eur Respir J. 2010;36:1185-206.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-specific Risk of Tuberculosis in Patients with Rheumatoid Arthritis Treated with Anti-TNF Therapy: Results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:522-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Biologics and Infections: Lessons from Tumor Necrosis Factor Blocking Agents. Infect Dis Clin North Am. 2011;25:895-910.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculous Peritonitis: A Surgical Dilemma. South Med J. 2009;102:94-5.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of Intestinal Tuberculosis by Polymerase Chain Reaction on Endoscopic Biopsy Specimens. Am J Gastroenterol. 1994;89:2248-9.
- [PubMed] [Google Scholar]
- Imaging of Gastrointestinal and Abdominal Tuberculosis. Eur Radiol. 2004;14:E103-115.
- [CrossRef] [PubMed] [Google Scholar]
- Intestinal Tuberculosis and Pulmonary Tuberculosis. Ileal Resection and PCR for the Diagnosis [Article in Spanish] An Med Interna. 2004;21:362-3.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic Dilemma of Abdominal Tuberculosis in Non-HIV Patients: An Ongoing Challenge for Physicians. World J Gastroenterol. 2006;12:6371-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peritoneal Tuberculosis: Laparoscopic Patterns and its Diagnostic Accuracy. Am J Gastroenterol. 1992;87:109-12.
- [PubMed] [Google Scholar]
- A Case of Tuberculous Peritonitis in the United States in a Patient with Rheumatoid Arthritis Treated with Adalimumab. J Clin Rheumatol. 2010;16:135-7.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the Treatment of Tuberculosis and Latent Tuberculosis Infection. JAMA. 2005;293:2776-84.
- [CrossRef] [PubMed] [Google Scholar]








