Translate this page into:
Morgagni-Larrey Diaphragmatic Hernia Masquerading as Calculous Cholecystitis – A Googly Tackled Laparoscopically

*Corresponding author: Dr. Prachi Praveen Agrawal, Department of General and Laparoscopic Surgery, Dr. L H Hiranandani Hospital, Mumbai, Maharashtra, India. prachiag1996@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Agrawal PP, Joshi AS. Morgagni-Larrey Diaphragmatic Hernia Masquerading as Calculous Cholecystitis – A Googly Tackled Laparoscopically. Int J Recent Surg Med Sci. 2024;10:129-33. doi: 10.25259/IJRSMS_38_2024.
Abstract
Sometimes, during a laparoscopy, the operating surgeon may be confronted with an unexpected, previously undiagnosed secondary surgical condition. In such cases, if the incidentally diagnosed condition warrants emergency surgical therapy, the surgeon must do it, albeit with some modifications. Herein, the authors describe a patient with gallstone disease who was taken up for laparoscopic cholecystectomy (LC). However, the initial laparoscopy revealed a complicated anterior diaphragmatic hernia. It was duly repaired in the same sitting, with some alterations in approach.
Keywords
Cholecystectomy
Complicated
Diaphragmatic hernia
Gall stone disease
Incidentally
Laparoscopic
Undiagnosed.
INTRODUCTION
Diaphragmatic hernia (DH) is caused because of the prolapse of abdominal contents into the thoracic cavity through a defect in the diaphragm. They lead to many respiratory, cardiac, and abdominal complications. DH can be either congenital or acquired. Congenital DHs are the most common type and usually make up more than 80–90% of all cases.[1] These are caused by a failure in the closure of the pleuro-peritoneal membrane during embryonic development. Amongst congenital DHs, the defect is mostly seen on the left posterolateral side of the diaphragm. This is because of the underlying liver supporting the right side of the muscle.[2] These DHs account for about 84% of congenital hernias and are known as ‘Bochdalek hernias.[3] Other forms include the ‘Morgagni hernias’, which comprise only 13% of the total patient load of congenital DHs, while central herniation makes is the rarest form of congenital DHs. Most DHs are preoperatively diagnosed. However, some are diagnosed incidentally and unexpectedly.
CASE REPORT
A 74-year-old female patient presented to the surgery outpatients department (OPD) with a complaint of pain in the epigastrium and right upper abdomen for 7 days. She had no other symptoms. The pain was dull, aching, and non-radiating in nature and did not have any aggravating or relieving factors. She had first consulted her family practitioner, who prescribed pain-relieving and anti-acidity medicine (Paracetamol and Pantoprazole), which did not provide any relief. She was advised to get an ultrasound (USG) scan of her abdomen, which Then revealed multiple gallbladder stones. The rest of the abdomen was normal. A diagnosis of calculous cholecystitis was thus made, and she was advised to undergo surgery–LC, for the same. After a due investigational workup and confirmation of fitness for general anesthesia, she was taken up for surgery. During the laparoscopy, she was found to have a left anterior diaphragmatic hernial defect. A large stretch of the greater omentum and part of the circumference of mid transverse colon was incarcerated into it [Figure 1a,b]. Immediately, the legs of the patient, who was originally in the supine state, were straightened and split. An extra 5 mm trocar was inserted into the left upper abdomen over and above the standard 4 trocars inserted for the LC. The surgeon moved in between the patient’s legs, and a second monitor, placed above the patient’s left shoulder facing the operating surgeon, was connected to the optic chain. The incarcerated contents were gently reduced completely [Figure 1c,d,and 2a]. The defect was then suture closed by no. 1-0 barbed suture (V-loc®) in a simple continuous fashion [Figure 2b,c,d and 3a,b]. A 15 X 12 cm dual mesh (Proceed®) was then introduced into the abdomen, placed optimally over the suture line, and fixed to the surrounding diaphragm with multiple simple 2-0 Prolene® sutures [Figure 3c,d]. The cholecystectomy was then carefully performed, taking due care not to open the gall bladder. The specimen was retrieved in a plastic bag, pneumoperitoneum desufflated, trocars removed, and port sites suture closed. The patient had an uneventful post-operative recovery. On her post-operative OPD follow-up on the 10th day, all her wounds had healed well. At the time of writing this paper, a telephonic interview was conducted with her. Eight months after her surgery, she continues to be asymptomatic.
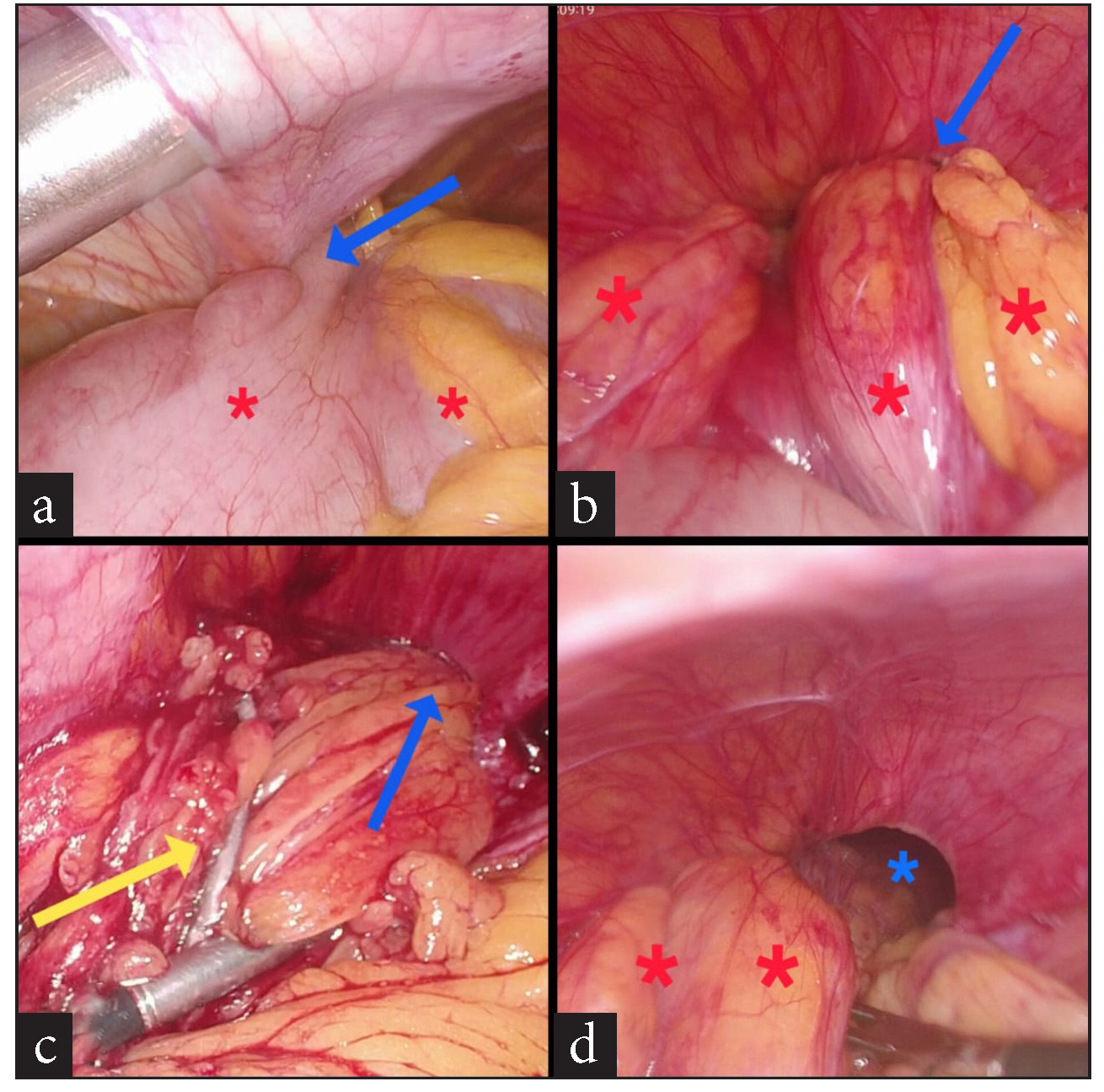
- (a) Part of circumference of mid transverse colon (red asterisks) trapped within diaphragmatic defect (blue arrow), (b) entrapped large chunks of greater omentum (red asterisks) within diaphragmatic defect (blue arrow), (c) incarcerated omentum being reduced (yellow arrow) from the defect (blue arrow), (d) 1st view of defect (blue asterisk) along with partially reduced omentum (red asterisks).
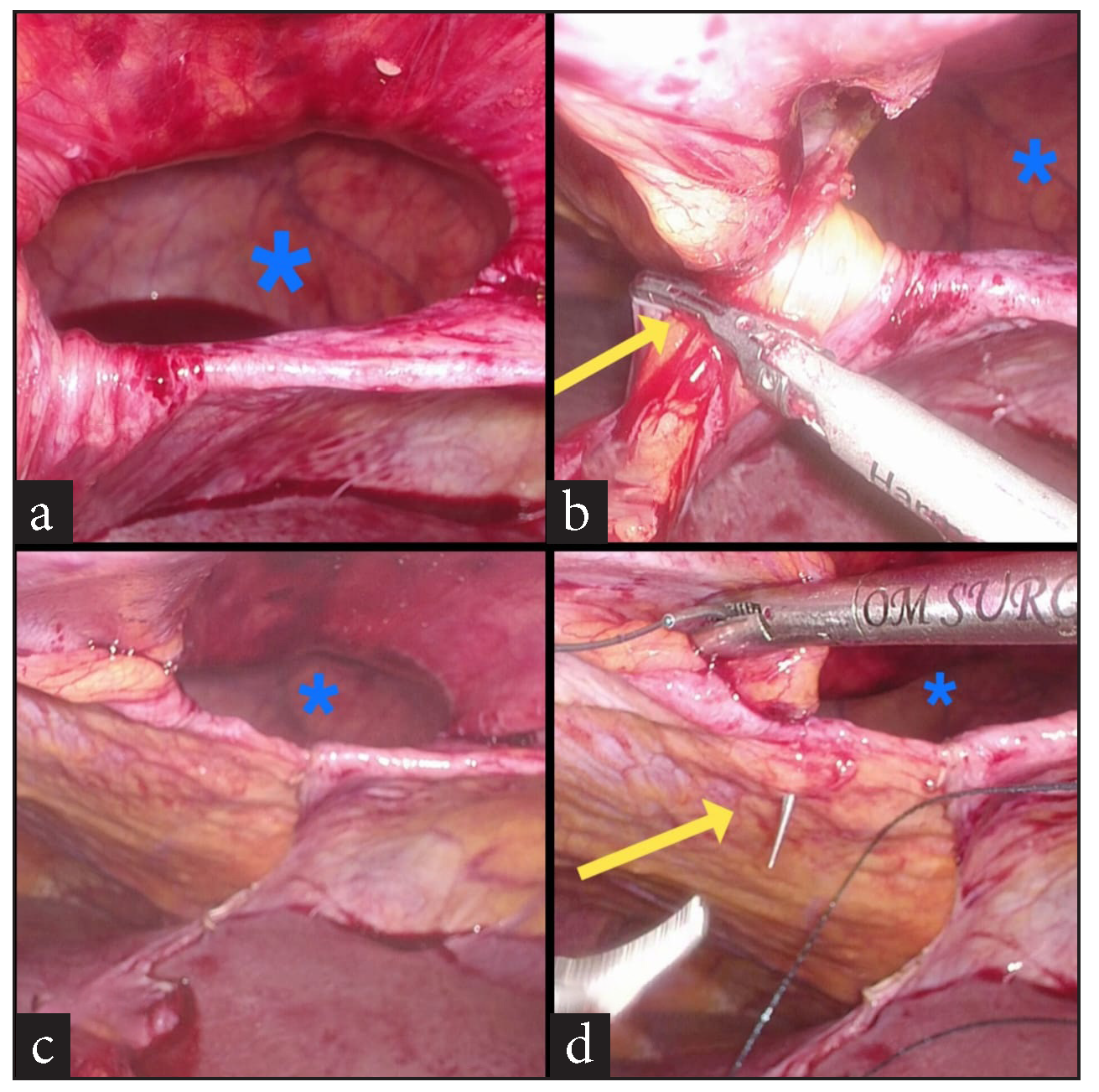
- (a) Diaphragmatic hernial defect (blue asterisk) after complete reduction of contents, (b) Diaphragmatic hernial defect (blue asterisk), division of falciform ligament (yellow arrow) to make way for the mesh, (c) Diaphragmatic hernial defect (blue asterisk), post division of the falciform ligament, (d) Diaphragmatic hernial defect (blue asterisk), suture closure of defect (yellow arrow) initiated.
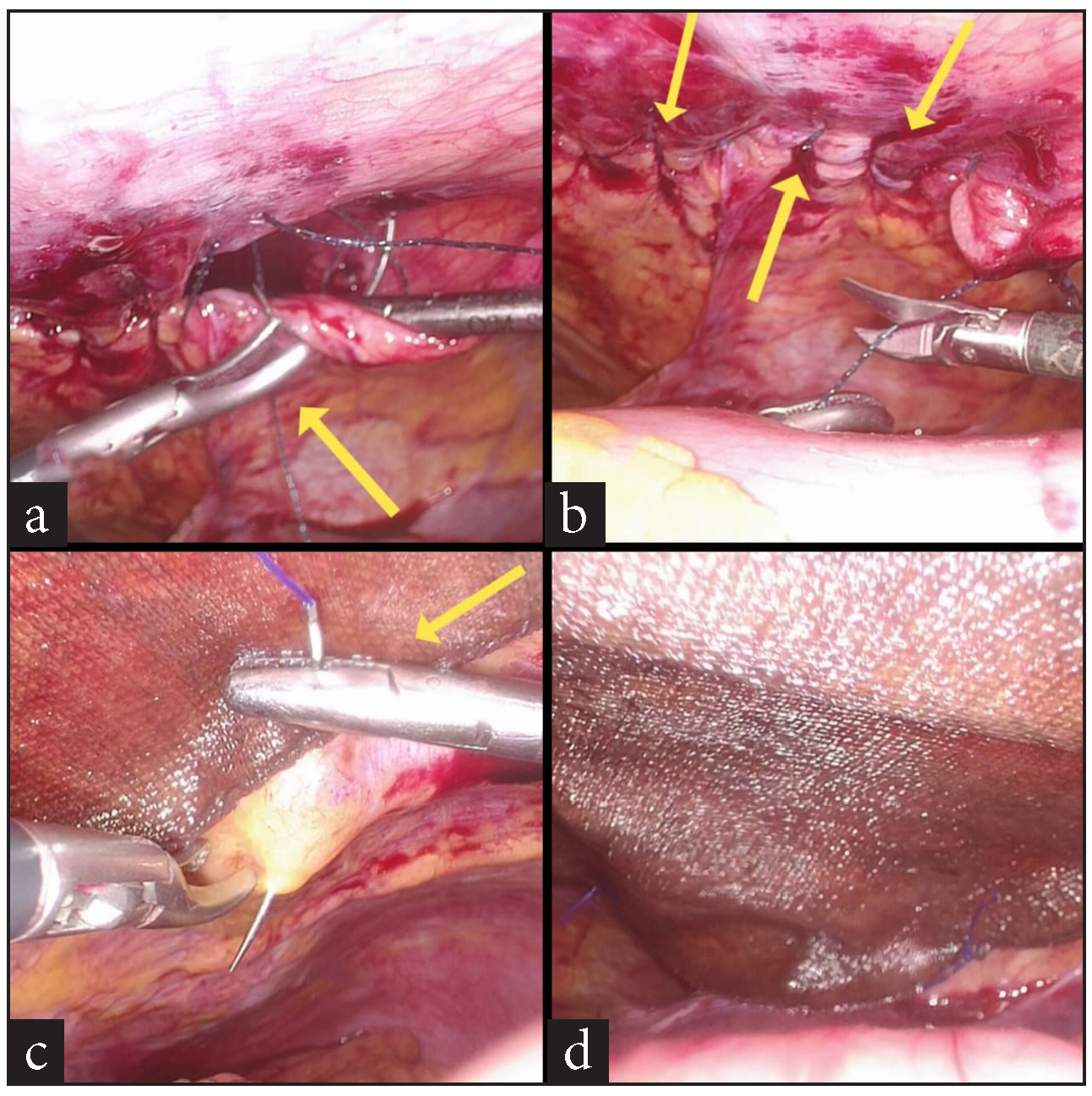
- (a) Suture closure of hernial defect in progress (yellow arrow), (b) the completed suture line (yellow arrows), (c) suture fixation of composite mesh to diaphragm (yellow arrow) over the suture line, (d) the end result.
DISCUSSION
Acquired DHs occur more commonly in adults. They are usually the result of blunt traumas or penetration injuries to the muscle, which cause increased pleuroperitoneal pressure, resulting in an anatomic orifice at the weak fusion sites of the diaphragm and lead to an upward protrusion of upper abdominal content.[4] The trauma could be of both iatrogenic or non-iatrogenic type. Even patient-affiliated treatment procedures, like–any cardiothoracic surgical procedure- chest tube misplacement, could result in an iatrogenic DH. Rarely, even a life-saving procedure such as a cardiopulmonary resuscitation could cause an injury to the diaphragm and inevitably result in a DH. While most surgical injuries are diagnosed and repaired intraoperatively, some of them can be missed and can manifest for months or, at times, years after the initial traumatic event. The longest documented delay in presentation is reported to be 35 years.[5] Iatrogenic DHs have been observed in literature following procedures such as esophagectomy, gastrectomy, LC, hepatectomy, and nephrectomy. The reported incidence of post-traumatic DHs ranges from 0.8% to 5%, depending on the source.[6]
The protrusion of the abdominal contents into the thoracic cavity through the DH (congenital or acquired) would cause a patient to complain about swelling, chest pain, and dyspnea. While intestinal symptoms may manifest as abdominal pain and fullness mainly after meals, nausea, and vomiting, patients could come with even obstructive symptoms or dysphagia. The clinical presentation of DH is variable, ranging from asymptomatic to respiratory, cardiac- and bowel-related complications. Depending on the organ system that is involved and protruding through the so-called defect, mortality rates can reach as high as 25%.[7] The clinical diagnosis of DH is grueling because of the eclectic variety of its symptoms. Computed tomography (CT), due to its high sensitivity and specificity, is the preferential modality to diagnose DH.
A Morgagni hernia, originating from the sternocostal trigone, is a congenital defect between the costal and sternal parts of the diaphragm on its anterior side. Giovanni Battista Morgagni, the founder of pathological anatomy, first described it in 1761. Retrosternal, parasternal, substernal, or sub-costosternal hernia are all synonyms for Morgagni hernia. As a convention, any deficiency in the diaphragmatic muscle on its right side is referred to as a Morgagni hernia, and on its left side is called a Larrey hernia.[8] The ligamentum teres is the one that is used to indicate the medial line of the hernia on both sides. In fact, available literature reports Morgagni hernia to be more common in males in childhood and more common in women during adulthood.[8] Although Morgagni hernias are congenital, they have occasionally been identified incidentally on prior radiographs, suggesting that herniation may develop later from these congenital diaphragmatic defects. Factors such as trauma, obesity, pregnancy, chronic constipation, and chronic coughing are known to contribute to the occurrence of Morgagni hernias in approximately 41% of cases.
When symptomatic, patients with Morgagni-Larrey hernia (MLH) may have dyspnea, abdominal or chest pain, and constipation in approximately 36%, 37%, and 20% of the cases, respectively.[9] The symptoms of the patients are dependent on hernial sac contents, with the small or large intestine leading to obstruction, the stomach leading to postprandial abdominal discomfort or reflux disease, and the omentum probably leading to pain. Pulmonary complications like dyspnea and exercise intolerance might be dependent on the hernia size.[9] Approximately 30% of MLH patients are reportedly asymptomatic.[9] Morgagni hernias are most frequently diagnosed by a chest X-ray or a CT scan. When the scans are used together, the accuracy of diagnosis is up to 80–90%.[10] A MLH is a rare condition, particularly in adults, and there are no established guidelines for its repair. Surgical intervention is the preferred treatment for Morgagni hernia. Various surgical techniques have been described in the literature, including laparotomy, thoracotomy, laparoscopic, thoracoscopic, and robotic repair. Compared to the trans-thoracic approach, the trans-abdominal approach offers better visualization of the hernia and provides a more comprehensive view of the relevant abdominal structures during repair.[9] A Trans-abdominal approach allows one to check for hernia-associated intraabdominal pathologies or complications such as strangulation and perforation. Aside from the advantage mentioned, some authors actually advocate a trans-thoracic approach as it helps by providing a better overview during the dissection of the hernia sac from the mediastinal, pleural, and pulmonary structures. Though thoracotomy is the most widely used method in the world, since the first laparoscopic approach in 1997, it has been increasingly used by surgeons.[11] Georgacopulo first reported a successful laparoscopic repair of a Morgagni hernia in a child in 1997.[12] The three main surgical treatment options include primary suture closure, primary suture closure with mesh reinforcement, and mesh interposition to reconstruct diaphragmatic integrity without relying on sutures for primary closure of the hernia. There is no established consensus on the superiority of direct repair or mesh repair. According to Ben-Yaacov et al., mesh repair is recommended when the defect area exceeds 20–30 cm2.[12] Commonly used materials for repairing such defects include biological meshes, dual meshes, or polypropylene meshes. To minimize adhesion between the mesh and intra-abdominal organs, techniques such as covering the mesh with peritoneum, omental fat, or falciform ligament flaps have been proposed. However, a significant drawback of mesh repair is the placement of tacks. As the diaphragm is particularly thin, especially in the medial region near critical structures, this could result in life-threatening injuries. To prevent such complications, primary sutures are preferred when operating near the pericardium.[10] If a mesh is used, it should adequately cover the defect with a 3–5 cm overlap on all sides for safety. There is also debate about whether the hernia sac should be removed. While leaving it intact may increase the risk of postoperative seroma formation, removing it could cause circulatory and respiratory complications by damaging mediastinal structures. Robotic surgery has been employed in a limited number of cases for diaphragmatic hernia repair, but current evidence is insufficient to establish it as the gold standard procedure for this condition. A review of recently published case reports on MLH is summarised [Table 1].
| Author/s | Journal/Yr of publication | Type of paper/no. of pts. | Diagnosed preop/intraop | Action taken | Mode of surgery (open/lap/thoracotomy/VATS/robotic) |
|---|---|---|---|---|---|
| Sharma A. et al.[13] | Cureus-2023 | Case report/1 | Preoperatively | Primary diaphragmatic hernia repair | open |
| Kuikel S. et al.[14] | International journal of surgery-2021 | Case report /1 | Preoperatively | Primary diaphragmatic hernia repair | Laparoscopic |
| Ağalar C. et al.[15] | Turkish journal of surgery-2019 | Case series/5 | Preoperatively | Primary Diaphragmatic hernia repair with (1)/without meshplasty (4) |
3-Laparoscopic (1 has recurrence after 7 months- repaired by open technique with meshplasty) 2-open |
| Supomo S. et al.[16] | Turkish journal of surgery-2022 | Case report/1 | Preoperatively | diaphragmatic hernia repair with dacron patch | Laparotomy + Thoracotomy |
| Christopher Adereti. et al.[17] | Cureus - 2023 | Case report/1 | Intraoperatively–while performing lap Sleeve gastrectomy | Not repaired | |
| Ali Hassan et al.[18] | International Journal of surgery case reports- 2019 | Case report/1 | Intraoperatively–while performing lap Nissen’s fundoplication | Primary diaphragmatic hernia repair | Laparoscopic |
| Racem Trigui et al.[19] | International Journal of surgery case reports- 2024 | Case Report/1 | Intraoperatively while performing elective LC | Primary diaphragmatic hernia repair | Laparoscopic |
VATS: Video assissted thoracoscopic surgery, LC: Laparoscopic cholecystectomy
CONCLUSION
As seen in this report, sometimes, unexpected and unanticipated events happen, or a previously undiagnosed condition becomes apparent during surgery. One is confronted with an event where one has to change/alter/modify one’s plan of action to give the best, minimally invasive surgical care to the patient. Also, as seen here, even a previously undiagnosed incarcerated diaphragmatic hernia can be effectively surgically repaired with some ergonomic modifications in the operating room, provided a good, well-equipped setup is ably complemented by advanced laparoscopic skills.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Incidental Finding of Right-Sided Idiopathic Spontaneous Acquired Diaphragmatic Hernia. Cureus. 2021;13:e15793.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diaphragmatic Hernia Masquerading as a Pulmonary Metastasis. Ann R Coll Surg Engl. 2015;97:e27-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Congenital Diaphragmatic Hernia. Radiopaedia.org. [accessed 2024 August 15]. Available from: https://doi.org/10.53347/rID-1161
- Emergency Surgery Due to Diaphragmatic Hernia: Case Series and Review. World J Emerg Surg. 2017;12:23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diaphragmatic Hernia an Uncommon Complication of Left Hemicolectomy for Colonic Adenocarcinoma. J Surg Res. 2021;4:197-201.
- [CrossRef] [Google Scholar]
- Surgical Approach in Management of Posttraumatic Diaphragmatic Hernia: Thoracotomy Versus Laparotomy. Case Rep Surg. 2020;2020:6694990.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diaphragmatic Hernia Presenting as Acute Gastric Outlet Obstruction: A Rare Complication of Left Lower Lobectomy. Cureus. 2022;14:e26544.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Laparoscopic Approach in the Treatment of Morgagni Hernia. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:514-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Morgagni-Larrey Diaphragmatic Hernia Repair in Adult Patients: A Retrospective Single-Center Experience. Hernia. 2021;25:479-89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Morgagni’s Hernia: Analysis of 21 Patients with Our Clinical Experience in Diagnosis and Treatment. Indian J Surg. 2018;80:239-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Morgagni-Larrey Hernia Correction by Laparoscopic Surgery. Eur J Pediatr Surg. 1997;7:241-2.
- [CrossRef] [PubMed] [Google Scholar]
- Repair of a Recurrent Symptomatic Hernia through the Foramen of Morgagni: A Case Study and Review of the Literature. J Surg Case Rep. 2020;2020:rjaa230.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An Incidental Finding of Morgagni Hernia in an Elderly Female and its Successful Management: A Rare Case Report and Review of Literature. Cureus. 2023;15:e42676.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Morgagni Hernia in Adult: A Case Report. Int J Surg Case Rep. 2021;85:106286.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adult Morgagni Hernia: A Single Center Experience of 5 Cases and Review of Literature. Turk J Surg. 2019;35:321-24.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Rare Adult Morgagni Hernia Mimicking Lobar Pneumonia. Turk J Surg. 2022;38:98-100.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Case Report and Literature Review on Incidental Morgagni Hernia in Bariatric Patients: To Repair or not to Repair? Cureus. 2023;15:e39950.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidental Morgagni Hernia Found During Laparoscopic Repair of Hiatal Hernia: Case Report & Review of Literature. Int J Surg Case Rep. 2019;57:97-101.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidental Finding and Successful Management of Larrey’s Hernia during Laparoscopic Cholecystectomy: Case Report. Int J Surg Case Rep. 2024;114:109149.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]








