Translate this page into:
Effect of Midodrine in Non-azotemic Liver Cirrhosis Patients with Refractory or Recurrent Ascites

*Corresponding author: Dr. Ningthoukhongjam Reema, M.D., Medicine, Department of Medicine, Regional Institute of Medical Sciences, Lalambung, Imphal West, Manipur, India. thangjamreema@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: M S Ajaygowda, Reema N, Gautam Singh T, Romeo Singh K, Theja PV, Krishnappa N, et al. Effect of Midodrine in Non-azotemic Liver Cirrhosis Patients with Refractory or Recurrent Ascites. Int J Recent Surg Med Sci. 2024;10:79-87. doi: 10.25259/IJRSMS_11_2024
Abstract
Objectives
To study the effectiveness of midodrine in treatment of refractory, recurrent ascites in nonazotemic liver cirrhosis patients.
Material and Methods
This is a facility-based open-label parallel design randomized controlled trial conducted at the Regional Institute of Medical Sciences (RIMS), Imphal. All patients above the age of 18 with non-azotemic liver cirrhosis with refractory or recurrent ascites patients attending medicine out patient department (OPD), liver clinic, and those admitted to the medicine ward, RIMS, Imphal, were enrolled. After getting informed consent, the patients were allocated to standard medical therapy (SMT) with the midodrine group (group A) and the SMT group (group B). Since there were two treatment options involved, a block size of four was used. Possible treatment allocation within each block was (1) AABB, (2) BBAA, (3) ABAB, (4) BABA, (5) ABBA and (6) BAAB. Both the study participants and the investigator were double-blinded. Mean arterial blood pressure, weight, frequency and volume of indicated large volume paracentesis (LVP), volume of urine in 24-hour, Estimated glomerular filtration rate (eGFR) by Cockcroft-Gault equation, and Child-Pugh classification score were calculated and recorded. Complete blood counts (CBC), liver function test (LFT), coagulation profile (prothrombin time (PT) and international normalized ratio (INR)), kidney function test (KFT), serum lipid profile, serology (HbsAg, Anti-hepatitis C virus (HCV) Ab, human immunodeficiency virus (HIV) 1&2), antinuclear antibody (ANA), ascitic fluid study, urine analysis, chest X-ray, Electrocardiogram (ECG) and echocardiography (patients with coronary artery disease, valvular heart disease with left ventricular (LV) systolic dysfunction or cardiomyopathy were excluded) were also done. computed tomography (CT) abdomen and upper gastrointestinal (GI) endoscopy (if indicated) were considered. The analysis was done using SPSS (trial version 23) software. A p-value < 0.05 was considered statistically significant.
Results
The present study enrolled 40 non-azotemic liver cirrhosis with refractory or recurrent ascites patients. In this study, after one month of treatment, there was a significant increase in urine output in the midodrine group compared with the SMT group (p-value 0.006). There was no statistical difference in model for end stage liver disease (MELD) scores after treatment among the groups. Mean arterial pressure (MAP), urine output, and glomerular filtration rate (GFR) were significantly higher in the midodrine group compared to the standard medical therapy (SMT) group after one month of treatment and were statistically significant and different.
Conclusion
The results of this randomized controlled trial (RCT) suggest that adding midodrine drug to the SMT group improves the systemic hemodynamics in non-azotemic cirrhotic patients with ascites, and it is also effective in lowering the body weights of the patients by decreasing the fluid accumulation. More clinical trials need to be conducted among a large number of patients before midodrine can be recommended for use in the patients.
Keywords
Liver cirrhosis
MELD score
Midodrine
Non-azotemic
Refractory ascites
INTRODUCTION
The most common sequelae of cirrhosis are ascites, hepatorenal syndrome, hepatic encephalopathy (HE), and upper gastrointestinal hemorrhage. Development of ascites predicts poor prognosis and lower standard of living (quality of life).[1] Such patients with ascites become more vulnerable to complications from bacterial peritonitis, hyponatremia, and hepatic hydrothorax and need diuretic therapy.[2] The prognosis worsens when ascites become resistant to therapy. Without a liver transplant, about 40–60% of patients are able to survive for two years.[3] Ascites progress to refractory ascites in about 5–10%, and within 6 months, the mortality rate is 50%. According to the International Ascites Club, refractory ascites cannot be mobilized or recur following large-volume paracentesis and cannot be adequately controlled by medical treatment.[4] Recurrent ascites are defined by frequent hospital admissions (more than three times per year) brought by the reaccumulation of ascites.[5]
Clinically, ascites can be classified as (1) diuretic-resistant ascites, which are unresponsive to the maximum tolerable dose of DT (400 mg/day of spironolactone and 160 mg/day of furosemide) and (2) diuretic-intractable ascites, which occurs when complications (e.g., hepatic encephalopathy, renal dysfunction or electrolyte abnormalities) prevent the use of diuretics at the therapeutically effective dose.[6]
Liver cirrhosis leads to restricted portal flow (in refractory ascites), causing portal hypertension. It is considered the first phase. Nitric oxide and other local vasodilators are released, which causes the splanchnic vessels to dilate.[7] Splanchnic arterial vasodilation reduces the amount of arterial blood in individuals with severe cirrhosis, making it challenging to maintain blood pressure. Associated neurohumoral activation and circulatory dysfunction[8] are reported. Vasoconstrictors and anti-natriuretic factors, such as the sympathetic nervous system and the renin-angiotensin-aldosterone system, are activated to compensate for this condition, leading to salt and water retention.[8] Vasodilation of the vessels and portal hypertension influence the permeability and pressure of the intestinal capillaries, which causes retained fluid in the abdominal cavity. Refractory ascites can develop for a variety of reasons, including markedly impaired renal excretion of free water, renal vasoconstriction, and sodium reabsorption.[9]
Options for refractory ascites are serial therapeutic paracentesis large volume paracentesis (LVP), transjugular intrahepatic portosystemic shunt (TIPS), peritoneovenous shunt, and liver transplantation.[10] Liver transplantation is the most successful treatment option but not always feasible due to its economic viability and lack of donors. In addition, some ascite patients are contraindicated for liver transplantation. As a result, non-transplant treatment alternatives for ascites that are refractory and recurring are currently receiving more attention.
It was reported that several vasoconstrictors, after administration to non-azotemic cirrhotic patients with ascites, circulatory, renal, and sodium excretion functions improved.[11,12] Specifically, midodrine, an oral a1-adrenergic agonist combined with octreotide and albumin, improved ascites control in individuals with refractory ascites.[13] Desglymidodrine (1 receptor agonist) is the active metabolite of midodrine, which raises blood pressure and causes an increase in vascular tone. It has no neural (diffuses weakly across the blood-brain barrier) or cardiac effects. It improves systemic and renal hemodynamics by reducing mesenteric vasodilatation in cirrhotic patients.[14]
There is a paucity of studies to evaluate the efficacy of midodrine in the management of refractory and recurrent ascites. So, this study was conducted to study the effect of midodrine in patients with liver cirrhosis with refractory or recurrent ascites.
MATERIAL AND METHODS
This is a facility-based open-label parallel design randomized controlled trial conducted in RIMS, Imphal, from 1st January 2021 to 1st July 2022. All patients with non-azotemic liver cirrhosis with refractory or recurrent ascites patients attending medicine outpatient department (OPD), liver clinic, and those admitted in the medicine ward, RIMS, Imphal, were enrolled. The census sampling method was used for data collection.
Inclusion criteria
All patients aged 18 and above, diagnosed as non-azotemic liver cirrhosis with refractory or recurrent ascites, and who were willing to participate were included in this study.
Exclusion criteria
All patients with gastrointestinal bleeding, hepatic encephalopathy or infection, those having hepatocellular carcinoma or portal vein thrombosis by Doppler study on the portal vein, and hepatorenal syndrome. Patients with a history of diabetes, renal or cardiovascular disease, or arterial hypertension, those having abnormal urine analysis, chest radiograph, or electrocardiograph, and patients not willing to participate were excluded from the study.
Sample size
Sample size (N) was calculated using the formula: Data taken from Singh et al.[14]
m1 = 14.75 (mean value of plasma renin activity in SMT group after 1 month),
m2 = 9.66 (mean value of plasma renin activity in SMT with midodrine group after 1 month),
s1 = 3.48 (SD for plasma renin activity in SMT group after 1 month),
s2 = 2.51 (SD for plasma renin activity in SMT with midodrine group after 1 month)
u = 80% =0.8 (Study power)
v = 0.05 =1.96 (α error)
The final sample size is 40 patients. (Including the dropout rate of 20%)
Randomization
After getting informed consent, the patients were allocated into SMT with the midodrine group (group A) and the SMT group (group B). Since there were two treatment options involved, a block size of four was used. Possible treatment allocations within each block were (1) AABB, (2) BBAA, (3) ABAB, (4) BABA, (5) ABBA and (6) BAAB. Both the study participant and the investigator were double-blinded.
Study procedure
A predesigned proforma including age, sex, etiology of cirrhosis, duration of disease, other comorbidity and medications and detailed physical examination of every patient was done. Meticulous examination and assessment for the following before and after one month of treatment with midodrine in tolerable dose was done. Mean arterial blood pressure [diastolic blood pressure +1/3 pulse pressure or 2/3 diastolic blood pressure +1/3 systolic blood pressure, weight, frequency of indicated LVP {> 50 ml/kg of ascites} and volume removed each time, volume of urine in 24-hour, eGFR by Cockcroft-Gault equation, Child-Pugh classification score were calculated and recorded. CBC, LFT, coagulation profile prothrombin time (PT) and International normalised ratio (INR)), Kidney function test (KFT), serum lipid profile, serology HBsAg, Anti Hepatitis C virus (HCV) Ab, Human Immunodeficiency virus (HIV) 1&2, Antinuclear antibody (ANA), ascitic fluid study, urine analysis, chest X-ray, ECG and echocardiography (patients with coronary artery disease, valvular heart disease with LV systolic dysfunction or cardiomyopathy were excluded) were also done. Computed tomography (CT) abdomen and upper gastrointestinal (GI) endoscopy (if indicated) were considered.
Outcome variables
Significant effect of midodrine on weight, mean arterial pressure, heart rate, GFR, urine output, urinary sodium, and MELD Score.
Intervention
The clinically confirmed and diagnosed cases of non-azotemic liver cirrhosis with refractory or recurrent ascites cases were divided into 2 groups as shown in Flow chart 1.
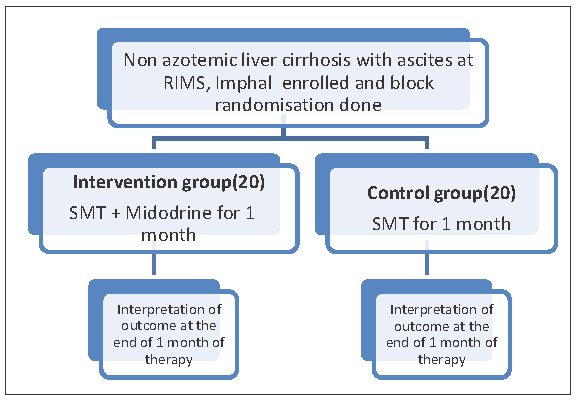
- Depicts the participants’ recruitment and study procedure.
Group A: patients in SMT [low sodium diet + diuretic therapy (loop diuretic in a dose 40–160 mg/day and distal acting diuretic in a dose100–400 mg/day)] + LVP as needed.
Group B: patients in SMT and midodrine tolerable dose for one month.
Operational definition
Chronic Alcoholic: Consumption of >3 standard drinks per day in males and >2 standard drinks per day in females for >5 years is defined as chronic alcohol use.[15]
Alcohol intake will be calculated in standard units/week
1 unit = one glass of wine = a standard measure of spirits (hard liquor) = half a pint of beer.
Chronic liver disease (CLD): Underlying CLD will be defined as either the presence of cirrhosis or chronic hepatitis of any etiology. The diagnosis of cirrhosis was based on clinical findings, biochemistry (low serum albumin, Aspartate aminotransferase (AST)/Alanine aminotransferase (ALT) ratio >1), imaging (heterogeneous echo texture of liver with irregular outline, altered liver size depending on etiology, portal vein > 13, portosystemic collateral), endoscopy (oesophageal varices) or documentation suggestive of prior decompensation.[16]
Refractory ascites was defined as ascites that cannot be mobilized or the early recurrence of which cannot be satisfactorily prevented by lack of response to sodium-restricted diet and high-dose diuretic treatment (400mg/day of spironolactone and 160mg/day furosemide) or development of diuretic-induced complications that preclude the use of an effective diuretic dosage.[17]
Recurrent ascites were defined as tense ascites that recurred on at least three occasions within 12 months despite standard treatment. The standard medical treatment was defined by the restriction of sodium, treatment with diuretics, and repeated LVP as needed.
Hepatorenal syndrome (HRS) is defined as having ascites and cirrhosis along with a serum creatinine level of less than 133 mmol/l (1.5 mg/dl), as well as not improving after at least two days of diuretic withdrawal and volume expansion with albumin (1 g/kg of body weight per day up to a maximum of 100 g/day) in the absence of shock, not receiving nephrotoxic drug treatment at the moment, not having parenchymal kidney disease as evidenced by proteinuria of less than 500 mg/day, absence of micro-haematuria (< 50 red blood cells per high-power field), and normal renal ultrasonography.[18]
Complete haemogram: Normal range for hemoglobin/Hb) (12–16 gm/dl), total leucocyte count (TLC) (4000–11,000/cumm) and platelet count (1.5–3.5 lakhs/cumm).[19] Anaemia[20] is Hb <12g/dL, leucopenia[21] is total count <4000cumm, and leucocytosis[22] is total count >11000cumm.
Thrombocytopenia[23] is platelets<1.5 lakhs/cumm.
Kidney function test: Normal range for Sr. urea (18–40 mg/dl), Sr. Creatinine (0.6–1 mg/dl), and Sr. Sodium (135–145 mEq/L).[24]
Liver function test: Normal range for total bilirubin (0.0–1 mg/dl), AST (5–40 IU), ALT (5–30 IU), and serum albumin (3.5–5.5 g/dl).[25]
International normalized ratio (INR) indicates the degree of hepatic anticoagulation measurement of INR is based on characteristics of the thromboplastin reagent used. Normal range INR: 0.9–1.2.[26]
Child-Pugh classification score of cirrhosis was used to assess the prognosis in the liver as shown in Table 1.[27]
| Parameters | Score | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Serum bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Serum albumin (g/dL) | >3.5 | 3-3.5 | <3 |
| International normalised ratio | <1.7 | 1.7-2.3 | >2.3 |
| Ascites | None | Easily controlled | Poorly controlled |
| Hepatic encephalopathy | None | minimal | Advanced |
Study tools
A complete haemogram was done by a hematology automated analyzer, LFT by the enzymatic analyzer, PT and INR by haemostatics analyzer, KFT uses a kinetic method for serum urea and Jaffe’s method for creatinine, hepatitis C serology by Flaviscreen method, hepatitis B serology by Viruschek rapid test and HIV I & II serology by Retrogine HIV kit. Ultrasound whole abdomen, UGI endoscopy, and CT scan whole abdomen will be done only if indicated.
Statistical analysis
Analysis was done in SPSS (trial version 23) software. Percentages, proportion, mean ± standard deviation (Mean and SD), and Student’s T-test were used for statistical analysis. A p-value < 0.05 was considered statistically significant.
Approval of the research ethics board and informed consent: This study was approved by the Research Ethics Board, RIMS, Imphal. (Reference No- A/206/REB-Comm (SP)/RIMS/2015/701/43/2020).
RESULTS
The present study enrolled 40 non-azotemic liver cirrhosis with refractory or recurrent ascites patients, and the mean (SD) age in years in the intervention group (midodrine group) was 46.9 (±10.34) years and in the control group was 49.85 (±12.57) years. Between the two groups, baseline socio-demographic and clinical characteristics were given in Table 2, baseline clinical characteristics between the two groups were given in Table 3, and baseline biochemical characteristics between the two groups were given in Table 4. The majority of them were males (15,75%) and (14,70%) in the intervention group and control group, respectively. The distribution of baseline co-morbidities between the groups was almost similar in both groups. Most of the patients were chronic alcoholics (90%), whereas the distribution of tobacco usage and IV drugs at the baseline was similar and comparable. The hemoglobin percentage in the study was less than normal and was around 8.9 gm/dl in the midodrine group and 8.4 gm/dl in the SMT group. Total leucocyte count was around 8.4 (±2.62) thousand and 8.85 (±3.6) thousand in the midodrine group and SMT group, respectively, and the values are within the normal range. Platelet count was similar in both groups but was less than the normal range. Random blood sugar was 114 (±35.7) mg/dl in the midodrine group and 136.55 (±50.4) mg/dl in the SMT group; there was no significant difference between the groups. Baseline total bilirubin values were very high, 8.9 (±1.29) in the midodrine group and 8.4 (±1.35) in the control group. The serum albumin was 2.9 (±0.85 g/dl) and 2.75 (±0.71) g/dl in the midodrine group and SMT group, respectively, and there was no significant difference between the groups. The mean distribution of LFT parameters among two treatment groups. The mean serum glutamic oxaloacetic transaminase (SGOT) among the midodrine group was 114.5 u/l, and the SMT group was 136.55 u/l. The mean serum glutamic pyruvic transaminase (SGPT) level among the midodrine group was 39.75 u/l, and among the SMT group was 49.4 u/l. The mean ALP level among the midodrine group was 122.95 u/l, and among the SMT was 173.15 u/l. The mean GGT level among the midodrine group was 111.4 u/l, and among the SMT was 169.7 u/l. The mean serum globulin among the midodrine group was 3.05 g/dl, and the SMT group was 3.65 g/dl.
| Variable | Classes | Midodrine group(n=20) n(%) | Standard medical therapy group (n=20) n(%) | p-value^ |
|---|---|---|---|---|
| Mean age in years(SD) | 46.9 (±10.34) | 49.85 (±12.57) | 0.423$ | |
| Gender | Male | 15 (75%) | 14 (70%) | 0.72 |
| Female | 5 (25%) | 6 (30%) | ||
| Hypertension | Absent | 20 (100%) | 19 (95%) | 0.31 |
| Present | 0 (0%) | 1 (5%) | ||
| Diabetes | Absent | 15 (75%) | 17 (85%) | 0.42 |
| Present | 5 (25%) | 3 (15%) | ||
| Alcohol | No | 4 (20%) | 0 (0%) | 0.03 |
| Yes | 16 (80%) | 20 (100%) | ||
| Tobacco | No | 20 (100%) | 19 (95%) | 0.31 |
| Yes | 0 (0%) | 1 (5%) | ||
| IV drug user | No | 19 (95%) | 18 (90%) | 0.54 |
| Yes | 1 (5%) | 2 (10%) |
It shows nearly 90 percent of the study participants were chronic alcoholics, and there was a significant difference in the percentage of alcoholics among the groups, whereas the distribution of tobacco usage and IV drugs at the baseline was similar and comparable. SD: Standard Deviation, IV: Intravenous.
| Variable | Classes | Midodrine group (n=20) n(%) | Standard medical therapy group (n=20) n(%) | p-value^ |
|---|---|---|---|---|
| Pallor | Absent | 9 (45%) | 3 (15%) | 0.03 |
| Present | 11 (55%) | 17 (85%) | ||
| Icterus | Absent | 2 (10%) | 0 (0%) | 0.14 |
| Present | 18 (90%) | 20 (100%) | ||
| Oedema | Absent | 1 (5%) | 3 (15%) | 0.29 |
| Present | 19 (95%) | 17 (85%) | ||
| Body weight (kg) | - | 76.80 (6.79) | 73.85 (5.73) | 0.146 |
| Mean arterial pressure (mmHg) | - | 76.4 (4.16) | 77.45 (6.52) | 0.54 |
| Pulse rate in beats per minute | - | 76.95 (15.5) | 82.45 (14.2) | 0.252 |
| Respiratory rate (cycles per minute) | - | 18 (1.29) | 17.5 (1.27) | 0.227 |
The table showed a significant difference in pallor between the groups though not significant for icterus. Mean arterial pressure, pulse rate, and respiratory rate were similar in both groups. BMI: Body mass index
| Variable |
Midodrine group(n=20) Mean (SD) |
Standard medical therapy group (n=20) n(SD) | p-value |
|---|---|---|---|
| Haemoglobin (g/dl) | 8.9(1.29) | 8.4(1.35) | 0.290 |
| Total leucocyte count in Thousands | 8.4 (2.62) | 8.85(3.6) | 0.656 |
| Platelet count in Lakhs | 1.34(0.67) | 1.39(0.66) | 1.000 |
| Random blood sugar in mg/dl | 114.5(35.7) | 136.55(50.4) | 0.119 |
| Total bilirubin (mg/dl) | 8.9(1.29) | 8.4(1.35) | 0.049 |
| Direct bilirubin (mg/dl) | 8.4 (2.62) | 8.85(3.6) | 0.130 |
| Indirect bilirubin (mg/dl) | 1.34(0.67) | 1.39(0.66) | 0.012 |
| SGOT (u/l) | 114.5(35.7) | 136.55(50.4) | 0.013 |
| SGPT (u/l) | 39.75(12.5) | 49.40(21.95) | 0.096 |
| ALP (u/l) | 122.95(61.86) | 173.15(79.5) | 0.032 |
| GGT (u/l) | 111.40(111.29) | 169.70(138.7) | 0.151 |
| Total protein (g/dl) | 5.95(0.75) | 6.40(0.88) | 0.092 |
| Serum albumin (g/dl) | 2.90(0.85) | 2.75(0.71) | 0.550 |
| Serum globulin (g/dl) | 3.05(0.82) | 3.65(0.98) | 0.044 |
| Blood urea | 39.80(16.4) | 47.65(18.8) | 0.169 |
| Serum creatinine (mg/l) | 1.10(0.308) | 1.05(0.224) | 0.560 |
| Serum sodium (meq/l) | 130.55(5.5) | 130.2(4.85) | 0.856 |
| Serum potassium (meq/l) | 3.95(0.39) | 4.20(0.83) | 0.233 |
| Serum chloride (meq/l) | 103(6.9) | 95.7(3.88) | 0.000 |
| Urine sodium (meq/24 hr) | 157.35(27.8) | 127.65(43.4) | 0.014 |
| Prothrombin time (PT) | 30.40(10.2) | 22.65(10.1) | 0.021 |
| MELD score | 24.60(5.10) | 25.35(4.8) | 0.640 |
| Urine output in ml | 1105(395.30) | 1008(386.91) | 0.82 |
| Estimated glomerular filtration rate in ml/min(eGFR) | 93.90(21.02) | 97.15(29.59) | 0.691 |
SGOT: Serum glutamic oxaloacetic transaminase, SGPT: Serum glutamic pyruvic transaminase, ALP: Alkaline phosphatase, GGT: Gamma glutamyl transferase. In this table, there was a significant difference between the two groups for bilirubin, SGOT, ALP, globulin, and PT, while there was no significant difference for Hb, total leukocyte count (TLC), thrombocytopenia, Kidney function test (KFT), and MELD scores, MELD: Model for End stage liver diseases, SD: standard deviation.
Among the two groups, blood urea was 39.80 (16.4) and 47.65 (18.8) in the midodrine group and SMT group, respectively, but there is no significant difference between the groups. Serum creatinine was slightly elevated in both the groups and there was no significant difference between the groups. Serum sodium was slightly decreased in both groups.
Clinical parameters before and after one month of therapy among groups are shown in Table 5. A comparison of outcome variables after one month of therapy across groups was given in Table 6. MELD score was 24.60 (5.10) and 25.35 (4.8) in the midodrine group and SMT group, respectively, and there was no significant difference between the groups (p- 0.640), but after one month of treatment, there was a significant decrease in MELD scores in midodrine group, but there is no much difference in the SMT group compared to baseline.
| Variable |
Midodrine group(n=20) Mean (SD) |
Standard medical therapy group (n=20) Mean (SD) |
||||
|---|---|---|---|---|---|---|
| Baseline | After 1 month | p-value | Baseline | After1 month | p-value | |
| Body weight (Kg) | 76.80 (6.79) | 70.20 (6.62) | 0.000 | 73.85(5.73) | 74.45(5.86) | 0.230 |
| MELD | 24.60 (5.18) | 22.60 (5.81) | 0.042 | 25(4.74) | 25.15(4.79) | 0.720 |
| Mean arterial pressure (mmHg) | 76.40 (3.42) | 86.45 (5.70) | 0.001 | 77.45(6.52) | 79.90(7.36) | 0.110 |
| Urine output (ml/24 hours) | 1105 (395.3) | 1890 (401.18) | 0.000 | 1008(386.91) | 1005.57(404.943) | 0.974 |
| eGFR (ml/min) | 93.90 (21.02) | 137.55 (48.57) | 0.003 | 97.15(29.59) | 106.9(33.23) | 0.102 |
This study showed that in the midodrine group, there was a significant increase in body weight, MAP, urine output, and eGFR after the treatment, while there was no significant increase for the same in the standard medical therapy (SMT) group. MELD: Model for end stage liver diseases, eGFR: Estimated glomerular filtration rate, SD: Standard deviation.
| Variable | Midodrine group (n=20) Mean (SD) |
Standard medical therapy group (n=20) Mean (SD) |
p-value |
|---|---|---|---|
| Body weight in kilogram | 70.20 (6.67) | 74.04 (5.86) | 0.03 |
| MELD | 22.60 (5.81) | 25.35 (4.74) | 0.110 |
| Mean arterial pressure | 86.40 (5.707) | 79.90 (7.36) | 0.003 |
| Urine output | 1890 (401.34) | 1005 (404.54) | 0.000 |
| eGFR | 137.05 (48.8) | 106.90 (33.23) | 0.006 |
In this study, there is a significant increase in urine output in the midodrine group compared to the standard medical therapy (SMT) group. There is no statistical difference in MELD scores after treatment among the groups. mean arterial pressure (MAP), urine output, and GFR are significantly higher in the midodrine group compared to the SMT group after one month of treatment. MELD: Model for End stage liver disease, SD: Standard deviation, eGFR: Estimated glomerular filtration rate
GFR increased in both groups but was significantly increased only among the midodrine group (p=0.003). Baseline urine output in the midodrine group was 1105(395.30) ml/24 hr, and in the SMT group was 1008 (386.91), and the values were similar and were comparable. Urine output was significantly higher in the midodrine group after the treatment compared with the control group after one month of treatment (p-value 0.006). Among the midodrine group, there is a significant decrease in body weight (p=0.000) after the treatment, whereas in the SMT group, there is a slight increase in body weight, but it is not significant (p=0.230).
When MAP was compared before and after the treatment in two groups, there was a significant increase in MAP among the midodrine group, but there was an increase in MAP among the control group, which was not significant. After one month of treatment with midodrine compared to baseline, there is no significant increase in MAP among the control group.
DISCUSSION
Ascites occur in nearly 50% of cirrhotic patients at least within 10 years period[28], and refractory ascites occur in 5–10% of cases.[29] In cirrhosis, splanchnic arterial vasodilatation is predominant; therefore, arterial vasoconstrictors could be a treatment option.[30] Vasoconstrictors (noradrenaline, terlipressin, octreotide, and midodrine) are useful in the treatment of HRS. Midodrine hydrochloride increases effective arterial blood volume by causing splanchnic vasoconstriction and improves renal perfusion and glomerular filtration through selective a1-adrenergic agonist action.[30] Moreover, midodrine prevents dialysis-induced hypotension and improves systolic blood pressure due to its effects on autonomic nerves. No side effects, such as an increase in the volume of fluid filtered by dialysis or a change in body weight in these patients. Midodrine, along with octreotide, increases MAP, renal plasma flow, GFR, urine volume, and urine salt levels, with non-azotemic and decreases recurrence of hydrothorax and mild ascites. Midodrine has also been used for the treatment of autonomic dysfunction, such as postural orthostatic tachycardia syndrome.
A total of 40 non-azotemic liver cirrhosis with refractory or recurrent ascites were enrolled in the study and were equally randomized into the midodrine group (n=20) and SMT group (n=20). The mean (SD) age in years in the intervention group (midodrine group) was 46.9(±10.34) years, and in the control group was 49.85(±12.57) years, which was similar to the sample size of 39 and 40 used in Singh V et al.[29] and Kalambokis G et al.[11], respectively.
In the present study, the mean age of the study participants was 48.5 years, which was comparable with the studies by Singh V et al.[29] (47 years) and Kalambokis G et al.[11] (52 years). The majority of the study participants were males in our study and other studies as well.[29,11]
There was a comparison of MAP, body weight, MELD scores, 24-hour urine output, and glomerular filtration rate among the groups after one-month treatment of midodrine and SMT.
In a study by Kalambokis G et al.[11], a combination of midodrine and octreotide administered to 13 non-azotemic cirrhotic patients with ascites for 11 days significantly decreased cardiac index and heart rate and increased MAP, systemic vascular resistance, and GFR. Similarly, our study showed a significant increase in MAP in the midodrine group one month after treatment, but it did not change significantly in the SMT group, which was consistent with the findings by Tandon et al.[13] and Singh et al.[14]
In the present study, the baseline 24-hour urine output was 1105 ml and 1008 ml among the midodrine and SMT groups, respectively. After one month of treatment, in the midodrine group, there was a significant increase in urine output, while there was no significant increase in the SMT group. Similar results were observed in the Singh V et al. study.[14] The present study showed a significant increase in GFR in the midodrine group after one month of treatment, whereas in the SMT group, there was no significant increase in GFR, which was consistent with the study by Singh et al.[14]
Our study showed a significant decrease in body weight in the midodrine group, whereas, in the SMT group, there was no significant decrease in body weight, which might be explained by the decrease in fluid accumulation by midodrine. This finding was at par with that of Ali et al. study.[31]
There was a significant decrease in MELD score in the midodrine group after one month of treatment but not in the SMT group, which was similar to the study by Tandon P et al.,[13] whereas Kalambokis G et al.[11] reported no significant decrease in MELD score.
Limitations
More clinical trials need to be conducted among a large number of patients before midodrine can be recommended for use in the patients.
CONCLUSION
The results of this randomized controlled trial suggest that adding midodrine drug to the SMT improves the systemic hemodynamics in non-azotemic cirrhotic patients with ascites, and it is also effective in lowering the body weights of the patients by decreasing fluid accumulation.
Ethical approval
This research/study was approved by the Institutional Review Board at RIMS, Imphal, number A/206/REB-Comm (SP)/RIMS/2015/701/43/2020, dated 8th February 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
REFERENCES
- Bacterial Infections, Sepsis, and Multiorgan Failure in Cirrhosis. Semin Liver Dis. 2008;28:26-42.
- [CrossRef] [PubMed] [Google Scholar]
- Management of Refractory Ascites and Hepatorenal Syndrome. Curr Gastroenterol Rep. 2011;13:17-25.
- [CrossRef] [PubMed] [Google Scholar]
- Transjugular Intrahepatic Portosystemic shunt for Refractory Ascites: A Meta-Analysis of Individual Patient Data. Gastroenterology. 2007;133:825-34.
- [CrossRef] [PubMed] [Google Scholar]
- The Management of Ascites in Cirrhosis: Report on the Consensus Conference of the International Ascites Club. Hepatology. 2003;38:258-66.
- [CrossRef] [PubMed] [Google Scholar]
- A New Method for Therapeutic Paracentesis: The Automated Low Flow Pump System. Comments in the Context of the History of Paracentesis. J Hepatol. 2013;58:850-2.
- [CrossRef] [PubMed] [Google Scholar]
- Nitric Oxide as a Mediator of Hemodynamic Abnormalities and Sodium and Water Retention in Cirrhosis. N Engl J Med. 1998;339:533-41.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and Management of Patients with Refractory Ascites. World J Gastroenterol. 2009;15:67-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Definition and Diagnostic Criteria of Refractory Ascites and Hepatorenal Syndrome in Cirrhosis. International Ascites Club. Hepatology. 1996;23:164-76.
- [Google Scholar]
- A Meta-Analysis of Transjugular Intrahepatic Portosystemic Shunt Versus Paracentesis for refractory Ascites. J Hepatol. 2005;43:990-6.
- [CrossRef] [PubMed] [Google Scholar]
- The Effects of Chronic Treatment with Octreotide Versus Octreotide Plus Midodrine on systemic Hemodynamics and renal Hemodynamics and Function in Nonazotemic Cirrhotic Patients with Ascites. Am J Gastroenterol. 2005;100:879-85.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of a 7-day Treatment with Midodrine in Non-Azotemic Cirrhotic Patients with and Without Ascites. J Hepatol. 2007;46:213-21.
- [CrossRef] [PubMed] [Google Scholar]
- The Effect of 1 Month of Therapy with Midodrine, Octreotide-LAR and Albumin in Refractory Ascites: A Pilot Study. Liver Int. 2009;29:169-74.
- [CrossRef] [PubMed] [Google Scholar]
- Midodrine in Patients with Cirrhosis and Refractory or Recurrent Ascites: A Randomized Pilot Study. J Hepatol. 2012;56:348-54.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic Liver disease. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Refractory Ascites: Pathogenesis, Clinical Impact, and Management. Gastroenterol Hepatol (N Y). 2009;5:647-56.
- [PubMed] [PubMed Central] [Google Scholar]
- Hepatorenal Syndrome: Update on Diagnosis and Therapy. World J Hepatol. 2017;9:293-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Blood Groups and Red Cell Antigens. Bethesda (MD): National Center for Biotechnology Information (US); 2005. Table 1, Complete blood count. [accessed on 2024 Feb 7]. https://www.ncbi.nlm.nih.gov/books/NBK2263/table/ch1.T1
- Anemia Screening. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Leukopenia, Lymphopenia, and Neutropenia in Systemic Lupus Erythematosus: Prevalence and Clinical Impact--A Systematic Literature Review. Semin Arthritis Rheum. 2015;45:190-4.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombocytopenia. 2023. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Hemodialysis Key Features Mining and Patients Clustering Technologies - Scientific Figure on ResearchGate. 2014 Oct 16 [accessed 2014 Oct 21]. https://www.researchgate.net/figure/Kidney-function-test-features_tbl1_258387855
- Liver Function tests. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- International normalized ratio (INR) In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Use of the Child Pugh Score in Liver Disease. 2023. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- [Google Scholar]
- Fluid Retention in Cirrhosis: Pathophysiology and Management. QJM. 2008;101:71-85.
- [CrossRef] [PubMed] [Google Scholar]
- Noradrenaline and Albumin in Paracentesis-Induced Circulatory Dysfunction in Cirrhosis: A Randomized Pilot Study. J Intern Med. 2006;260:62-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute Effects of the oral Administration of Midodrine, an Alpha-Adrenergic Agonist, on Renal Hemodynamics and Renal Function in Cirrhotic Patients with Ascites. Hepatology. 1998;28:937-43.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical Study on the therapeutic Role of Midodrine in Non Azotemic Cirrhotic Patients with Tense Ascites: A Double-Blind, Placebo-Controlled, Randomized Trial. Hepatogastroenterology. 2014;61:1915-24.
- [PubMed] [Google Scholar]







