Translate this page into:
METHEMOGLOBINEMIA AFTER ISOLATED LIDOCAIN SPRAY: A RARE PHENOMENON
-
Received: ,
Accepted: ,
This article was originally published by Thieme Medical and Scientific Publishers Private Ltd. and was migrated to Scientific Scholar after the change of Publisher.
How to cite this article –Khandelwal P, Malani R, Kothari S et. al. Methemoglobinemia after Isolated Lidocaine Spray: A Rare phenomenon, IJRSMS, 2015;01(1): 5 – 7
Abstract
Methemoglobinemia is a state recognized by the increased production of met-hemoglobin, which is a form of oxidized hemoglobin, which is unable to bind oxygen. As a result the patient has a functional anemia, in which the remaining oxyhemoglobin has increased oxygen affinity, shifting the oxygen curve to left and perpetuating the impairment of oxygen delivery to tissues. Methemoglobinemia is a rare, but serious cause of hypoxemia, which can be difficult to recognize. It has been traditionally associated with the use of benzocaine class of anesthetic agents and FDA has issued warning for the use of benzocaine class of anesthetic agents for the same reason. It has been very seldom reported with the use of lidocaine class of agents alone. Through this case report we want to emphasize development of methemoglobinemia through the isolated use of lidocaine.
Keywords
Congenital Methemoglobinemia
Lidocaine toxicity
Acquired Methemoglobinemia.
INTRODUCTION
Methemoglobinemia is a rare but serious cause of hypoxemia, which can be difficult to recognize. There are acquired and inherited forms of Methemoglobinemia. Acquired methemoglobinemia is usually seen as a complication of a variety of medications, including anesthetics such as benzocaine and prilocaine (1). It is seldom reported with the isolated use of lidocaine. We present a rare case of a patient who developed methemoglobinemia after the use of lidocaine spray, along with a brief review of the literature.
CASE PRESENTATION
A 56-year-old woman with a previous medical history significant for colon cancer s/p colostomy was admitted to the neurology service for workup of a right middle cerebral artery stroke. The patient's labs result demonstrated an elevated cholesterol level of 238 mg/dL, LDL level of 154.3 and a Glycated hemoglobin level of 6.4. The remaining labs, including a hypercoagulable workup, were within normal limits. Given the patient's large cortical stroke, likely embolic in nature, a transesophageal echocardiogram (TEE) was ordered to look for a cardiac source of emboli.
As part of the preparation for the procedure, lidocaine 10% spray was used. Roughly 7-10 minutes after the use of the spray the patient began to complain of chest pain and was dyspneic. Vitals at this time demonstrated a heart rate of 83 bpm, blood pressure of 153/87 mmHg, respiratory rate of 26 breaths per minute, and an oxygen saturation of 72%. An EKG demonstrated normal sinus rhythm at rate of 86 bpm and ST depression of 1mm in the inferior leads II, III and AVF. Troponin was mildly elevated at a level of 0.067. An arterial blood gas was also drawn to evaluate the degree of hypoxemia. As labs were drawn it was incidentally noted to have an extremely dark color, which raised the suspicion of methemoglobinemia as a possible cause of the patient's signs and symptoms. Based on the patient's pulse oximetry, arterial blood gas, pO2 levels and clinical picture, a diagnosis of methemoglobinemia was made.
A weight-based dose of 75mg IV methylene blue was administered over a 5-10 minute period. The patient was extubated 18 hours later with a post extubation blood gas of pH 7.46 with O2 saturation of 99%. She was no longer in any respiratory distress and denied any chest pain.
DISCUSSION
Physiology:
Each day 0.5 to 3 percent of available hemoglobin is converted to methemoglobin, which constitutes the physiological levels found in the average person. This methemoglobin can be reduced by cytochrome b5 reductase (b5R) in an NADH-dependent reaction2. Methemoglobinemia is a state recognized by the increased production of methemoglobin, which is a form of oxidized hemoglobin, which is unable to bind oxygen (Figure A) (2). As a result the patient has a functional anemia, in which the remaining oxyhemoglobin has increased oxygen affinity, shifting the oxygen curve to left and perpetuating the impairment of oxygen delivery to tissue (3,4).
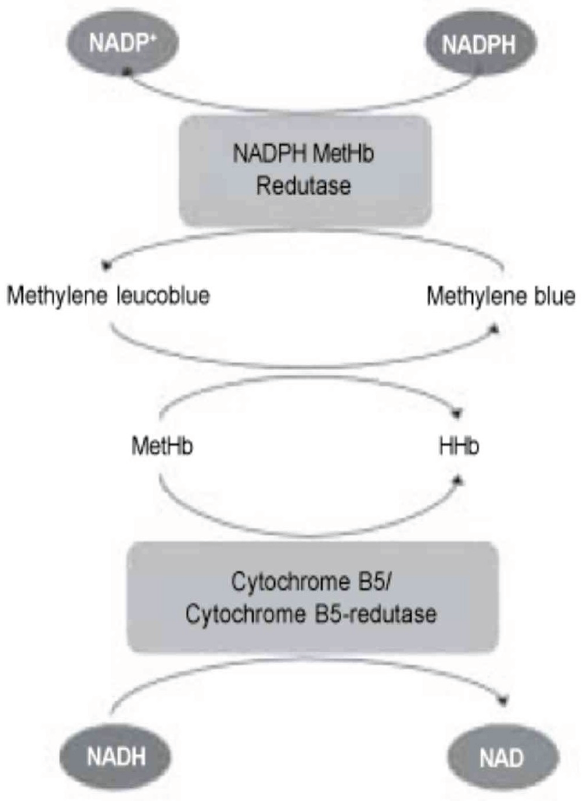
- Main Pathway of Methemoglobin Reduction
Pathology:
Methemoglobinemia is broadly classified in 2 categories: congenital and acquired. Congenital methemoglobinemia occurs due to deficiency of enzymes nicotinamide adenine dinucleotide (NADH) b5 reductase and nicotinamide adenine dinucleotide phosphate flavin reductase. Glucose-6-phosphate inhibits the oxidation of hemoglobin, so an individual with G6PD deficiency is more susceptible to the development of methemoglobinemia (4). Hemoglobin M disease is another congenital cause for development of Methemoglobinemia. Acquired methemoglobinemia is much more common and occurs on exposure to oxidizing agents. Common offenders are aniline dyes, nitrobenzene, nitrate, nitrite, benzocaine, prilocaine, dapsone, pyridium, nitric oxide, nitrous oxide and naphthalene (3,5).
Methemoglobinemia has also been documented, albeit uncommonly, by the use of EMLA cream (a cream used for topical purposes which constitutes a combination of prilocaine and lidocaine) (6).
Lidocaine alone has been shown as the cause of methemoglobinemia in very rare instances. A recent paper by Guy et al. showed only twelve subjects out of 242 cases of methemoglobinemia were related to lidocaine without the association of prilocaine or benzocaine. Of those twelve patients, seven were found to have an oxidative agent administrated concomitantly. Only three of the patients developed methemoglobinemia after appropriate clinical use of lidocaine, however none of the cases were tested for NADH deficiency (7).
Clinical symptoms and treatment. -
Clinically the patient may present with a wide variety of symptoms ranging from being asymptomatic to central or peripheral cyanosis, respiratory depression or dyspnea, altered consciousness, shock, seizures and possible death. Treatment is usually associated with administration of Methylene blue that converts the oxidized heme to its reduced state (8).
CONCLUSION
Methemoglobinemia is a rare condition and is well known to be associated with the Benzocaine class of local anesthetics. The FDA has issued warnings for the cautious use of benzocaine and related drugs. Through this case, we emphasize that methemoglobinemia can occur with the isolated used of lidocaine; therefore appropriate caution is warranted prior to its use in endoscopic procedures and other indications. Further, if a patient were to develop the above-mentioned signs and symptoms after the use of lidocaine, methemoglobinemia should be included in the differential diagnosis.
Conflict of Interest
NIL
REFERENCES
- Severe methemoglobinemia from topical anesthetic spray: case report, discussion and qualitative systematic review. CJEM. 2001;3:51-55.
- [CrossRef] [PubMed] [Google Scholar]
- Methemoglobinemia: from diagnosis to treatment. Revista Brasileira de Anestesiologia. 20082008;58(06):651-664.
- [CrossRef] [PubMed] [Google Scholar]
- Benzocaine and lidocaine induced methemoglobinemia after bronchoscopy: a case report. Journal of Medical Case Reports. 2008;2:16-20.
- [CrossRef] [PubMed] [Google Scholar]
- methemoglobinemia-susceptible patients: A case report and review of literature. Anesth Prog. 2004;51:24-7.
- [Google Scholar]
- Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-5.
- [CrossRef] [PubMed] [Google Scholar]
- Methemoglobinemia after using EMLA cream. Ned Tijdschar Geneeskd. 2013;157(29):A6206.
- [Google Scholar]
- Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108(03):837-845.
- [CrossRef] [PubMed] [Google Scholar]
- Cellular and Molecular Actions of Methylene blue in the Nervous System. NIH: Med Res Rev. 2011;31(01):93-117.
- [CrossRef] [PubMed] [Google Scholar]







