Translate this page into:
The Endoscopic Transsphenoidal Technique for Acromegaly: Evaluating Remission

*Corresponding author: Dr. Anmol Singh Randhawa, Department of Neurosurgery, Mahatma Gandhi University of Medical Sciences and Technology, RIICO Industrial Area, Sitapura, Jaipur, Rajasthan, India. dr.anmolrandhawa@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Randhawa AS, Sherpa TD, Srivastava A, Agrawal Y, Jain PK, Gill M, et al. The Endoscopic Transsphenoidal Technique for Acromegaly: Evaluating Remission. Int J Recent Surg Med Sci. 2024;10:102-13. doi: 10.25259/IJRSMS_35_2024.
Abstract
Objectives
The primary strategy for managing acromegaly involves transsphenoidal surgical intervention, with the achievement of post-surgical remission playing a pivotal role in patient prognosis. Factors such as pre-surgery GH (growth hormone) as well as insulin-like growth factor-1 levels, tumour size, invasion into the cavernous sinus and the extent of tumour resection outside the capsule have been recognised as crucial for determining remission outcomes.
Material and Methods
Between January 2017 and April 2023, 89 patients underwent endoscopic transsphenoidal surgery at the Mahatma Gandhi University of Medical Sciences and Technology in Jaipur. The study assessed preoperatively along with postoperative parameters such as overall remission, cavernous sinus invasion, the extent of resection beyond the tumour capsule, resection rates and GH levels before and after surgery. Remission rates were evaluated based on the consensus criteria from 2010.
Results
Of the 89 patients, 79 (88.8%) achieved complete tumour resection. Remission was observed in 70 patients (78.7%), with 61 of 78 patients (78.2%) having larger tumours (macroadenomas), 9 of 11 (81.9%) with smaller tumours (microadenomas), and 7 of 18 (38.9%) with tumours invading the cavernous sinus achieving remission. Remarkably, 68 of 72 patients (82.9%) who had a pseudocapsular resection entered remission. Patients with preoperative GH levels below 20 ng/mL saw an 87.5% remission rate, which dropped to 33.3% for those with levels above 60 ng/mL. The study found strong associations between remission rates and factors such as extra-pseudocapsular resection and cavernous sinus invasion, with a negative correlation to tumour size and volume.
Conclusion
The key determinants of remission include the extent of tumour invasion into the cavernous sinus and the completeness of tumour excision. Achieving optimal remission outcomes requires meticulous surgical removal of the tumour, including any residual fragments, with preoperative as well as postoperative GH levels serving as prognostic indicators of remission success.
Keywords
Acromegaly
Endoscopic
Growth hormone
Remission
Transsphenoidal
INTRODUCTION
Acromegaly is characterised by higher levels of GH (growth hormone) along with IGF-1 (insulin-like growth factor-1) that occurs due to pituitary tumour[1] and leads to distinctive facial and skeletal changes along with metabolic, cardiovascular and respiratory system complications.[2,3] This condition not only burdens individuals with numerous health complications but also elevates mortality rates.[4]
The primary objectives in the treatment of acromegaly are to regulate IGF-1 as well as GH secretion, curb tumour growth, along with preserving the functionality of pituitary hormones.[5] Normalisation of GH levels is essential for symptom relief and in bringing mortality rates closer to normative population figures.[2] Acromegaly has an incidence rate of five per million people annually, with treatment modalities encompassing surgery, radiation and medication.[6]
Surgery via the transsphenoidal method is often the first-line approach for treating acromegaly.[7–14] The effectiveness of microscopic surgery for removing pituitary adenomas varies, with success rates for microadenomas between 67% and 95% and for macroadenomas between 47% and 68%. The overall success rates span from 42% to 72%.[6,15–18] Recent findings indicate a growing preference for endoscopic transsphenoidal surgery in addressing pituitary adenomas. This technique offers an expanded view of the operation area, improving the surgeon’s ability to see the lateral aspects of the suprasellar compartment and cavernous sinus, which is particularly beneficial for larger tumours.[19–22] Yet, it remains to be determined if the endoscopic method is more effective or safer than the microscopic technique for this type of surgery.[5,20,23,24]
The neurosurgeon’s expertise and experience significantly influence the likelihood of a successful surgical outcome. While surgeries performed by a single surgeon can be more closely adapted to each patient’s needs, such an approach may suffer from small sample sizes and data collection inconsistencies. Conversely, registry data encompassing surgeries by various surgeons offers a wider scope of cases, enhancing the general applicability of the findings. In our study, three neurosurgeons carried out all procedures to minimise potential biases linked to individual surgeon’s experience levels.
The 2010 consensus recommendations describe remission as having normal IGF-1 levels that have been corrected for the patient’s age and sex as well as a random GH level of less than 1 ng/mL postoperatively or a GH level of less than 0.4 ng/mL following an oral glucose tolerance test.[25] Following an acromegaly surgery, reports of biochemical remission range from 34% to 83%.[6,21,26–30] Key factors identified as influencing remission include the average preoperative levels of GH and IGF-1,[7,26] the size of the tumour,[31–33] whether the cavernous sinus has been invaded,[7,34] the accomplishment of extra pseudo capsular resection,[35,36] and the experience of the surgeon.[9] Utilising the fourth-largest patient dataset to date, this study retrospectively reviews the early results and consequences of endoscopic surgery on the pituitary for patients with acromegaly in order to investigate the factors that influence biochemical remission.
MATERIAL AND METHODS
Patients
From January 2018 to December 2023, Mahatma Gandhi University of Medical Sciences and Technology in Jaipur conducted endoscopic transsphenoidal surgeries on 89 patients. These individuals, all diagnosed with GH-secreting adenomas, had their medical records carefully reviewed retrospectively, ensuring that informed consent was obtained from each one. Both neurological and endocrinological evaluations were performed before and after the surgeries. The information for this study was compiled from imaging studies and the patient’s medical records.
The study excluded patients under the following conditions:
-
-
Those who did not provide consent
-
-
Those deemed unfit for general anaesthesia
-
-
Those who were not available for follow-up
-
-
Those with a previous history of medical treatment or Gamma Knife radiosurgery
-
-
Those lacking essential biochemical data or MRI scans
-
-
Those who received a transcranial surgical approach instead
The diagnosis of acromegaly in these patients followed the clinical practice guideline, which involves IGF-1 levels that exceed the normal upper limit for the patient’s age and sex, coupled with the incapability to reduce GH levels to below 1.0 ng/mL after an OGTT (oral glucose tolerance test).[5]
In addition to pre- and post-surgery assessments by neuro-ophthalmologists and endocrinologists, the study paid close attention to any declines in visual acuity or visual field defects, categorising these as visual deficits. Moreover, endocrine evaluations prior to surgery involved measuring all adenohypophyseal hormone levels.
Following their surgeries, all patients were monitored for a minimum of three months, with an average follow-up duration of 12 months.
Endocrine analysis
All patients were subjected to hormonal evaluations both before and after their surgery, focusing on GH and IGF-1 levels, alongside an examination of the anterior pituitary function. This process included an oral glucose tolerance test following the measurements of the initial IGF-1 as well as GH level. The pre-surgery GH levels had been organised into four groups: 0–20, 20–40, 40–60 and over 60 ng/mL. The GH as well as IGF-1 levels detection was carried out using a specialised immunoradiometric assay kit, which identified the minimum detectable levels as 0.02 ng/mL for GH and 4.55 ng/mL for IGF-1. The assessment of GH levels immediately after surgery was scheduled for the morning of the third postoperative day and again at the third and sixth months.
In all patients, the assessment for hypopituitarism was performed before and after the surgery. The levels of various hormones, including FSH (follicle-stimulating hormone), LH (luteinising hormone), ACTH (adrenocorticotropic hormone), free T4, E2 (estradiol) or total testosterone, prolactin, TSH (thyroid-stimulating hormone) as well as serum cortisol had been computed using RIA (radioimmunoassay), usually conducted from 8:00 a.m. to 10:00 a.m. A deficiency in ACTH has been identified by peak cortisol levels of ≤ 18 µg/dL after a short Synacthen test or by a low morning cortisol level (< 5 µg/dL) in conjunction with a low-normal ACTH level (10–65 pg/mL). A deficiency of TSH had been indicated by a reduced normal TSH level (reference range, 0.4−4.1 µIU/mL) in conjunction with a minimal free T4 level (< 0.70 ng/dL). For those diagnosed with hypopituitarism, the onset of normal menopause had been determined by an FSH level above 30 mIU/mL, along with estradiol levels under 50 pg/mL. Premenopausal women with adequate levels of FSH/LH were deemed free of menstrual issues. In males, diminished testosterone levels accompanied by low-normal FSH/LH levels indicate the necessity for an assessment of central hypogonadism. Patients exhibiting symptoms indicative of water diuresis as well as polydipsia were evaluated for diabetes insipidus, which had been classified as transient (less than one month) or else permanent (three months or more) according to the desmopressin need. Individuals exhibiting hypopituitarism in both clinical and laboratory settings were administered hormone replacement therapy as necessary.
Image analysis
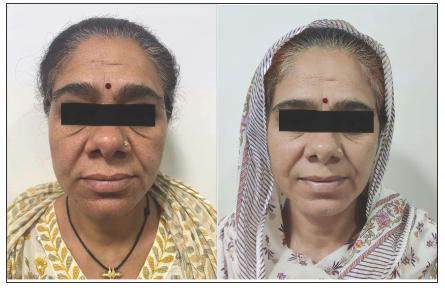
Magnetic Resonance Imaging (MRI) scans were conducted on all patients before their operations as well as during follow-ups at three months and one year. Before surgery, every case of pituitary adenoma was identified using preoperative MRI. Dynamic MRI scans were conducted in both sagittal and coronal perspectives, with a focus on the sella turcica and the surrounding parasellar area, both before and following the administration of gadolinium contrast, in order to obtain additional evaluation of these adenomas [Figures 1 and 2]. The size of the tumours was determined by measuring the largest diameter found in the preoperative MRI scans. Adenomas were categorised into two groups based on size: tumours less than 10 mm in diameter are known as microadenomas, and macroadenomas in those that are larger than 10 mm. Additional evaluations included checking for growth beyond the pituitary area, pressure on the optic chiasm and invasion into the cavernous sinus, all categorised using the Knosp classification. Tumours that surrounded the internal carotid artery were given a grade 4 classification based on their appearance in coronal T1-weighted images post-contrast.[37]
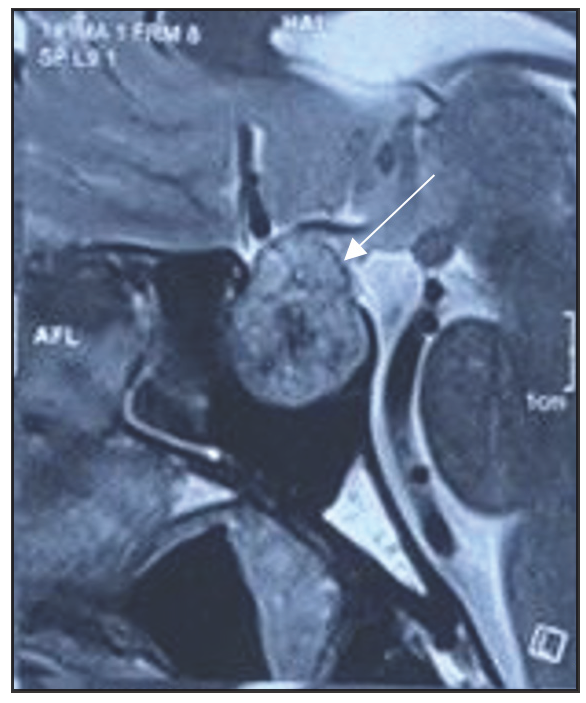
- Sagittal T2 WI MRI image (preop) showing well-defined mass lesion in sella (heterogeneously hyperintense), (white arrow) (original).
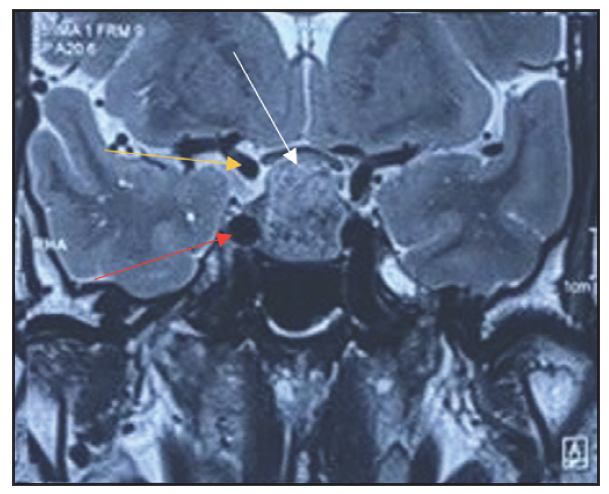
- Coronal T2 WI MRI image (preop) showing sellar mass with no para-sellar extension (white arrow), Right Cavernous segment of ICA (Red arrow), Right Supraclinoid segment of ICA (Yellow arrow) (Original)
Remission analysis
Remission evaluation adhered to the guidelines established in 2010, focusing on achieving normal IGF-1 levels adjusted for the individual’s sex and age, a post-surgery random GH level under 1 ng/mL or a GH level lower than 0.4 ng/mL following an oral glucose tolerance.[25] Oral glucose tolerance tests and IGF-1 level measurements were employed to evaluate remission status three months after the procedure, and these measurements were repeated every three months for the first year.
This process of assessing remission involved a comparative analysis of data before and after surgery. The analysis covered a range of factors, including overall remission rates, rates of postoperative resection, the incidence of cavernous sinus invasion, success in achieving extra-pseudocapsular resection and the comparison of GH levels pre- and postoperation. The study further segmented remission rates by the size of the adenomas (macro versus micro) and by different classifications of tumour volume. An additional layer of assessment was provided for cases presenting with or without cavernous sinus invasion. The evaluation also extended to patients who had undergone extra-pseudocapsular resection.
Statistical analysis
For the statistical analysis, SPSS version 13 was employed. To determine ranges, means, frequencies as well as distributions, descriptive statistical methods were applied. An independent t-test was utilised to assess the variances among measurements taken before as well as after surgery. To analyse the variations in postoperative IGF values and the rates of remission, the chi-square test (χ2) was utilised. A p-value of less than 0.05 was designated as the significance threshold. The relationship between the adenoma’s size along with the volume, the resection rates, the incidence of cavernous sinus invasion as well as the groups undergoing extra-pseudocapsular resection was examined using the Pearson correlation test.
Surgical technique
The surgical techniques utilised in this study were in line with those outlined in the current literature.[38,39] All surgeries were performed using a bi-nostril transseptal approach under endoscopic visualisation. Hemostatic material was applied to control nasal mucosa bleeding with minimal coagulation to maintain mucosal function as well as prevent postoperative synechiae. An anterior sphenoidotomy was conducted using a drill guided by anatomical landmarks such as the choana and sphenoid ostium. Septal differences were identified utilising a preoperative paranasal sinus CT (computed tomography) scan; the rostrum was crucial for midline identification. Employing a high-speed drill, the sellar floor was opened, revealing the bilateral cavernous sinus and superior intercavernous sinus. For GH adenoma surgery, meticulous removal of small remnants hidden by fibrin membranes was crucial, especially for tumours in challenging locations. Although extra-pseudocapsular excision was frequently challenging, it was typically successful in identifying the pseudocapsule.
In 82 cases, the pseudocapsule was identified, allowing for extra-pseudocapsular resection, while intracapsular resection had been accomplished in other cases. Resection of macroadenomas was done posteroinferiorly, laterally and anteroinferiorly; superior removal was done at the end to avoid premature cisternal collapse. Fibrotic adenomas were removed using an ultrasonic aspirator. The application of sharp, angled ring curettage in blind curettement was not established. After confirming the position of the interval carotid artery with intraoperative Doppler ultrasonography and neuronavigation, tumours in the cavernous sinus were removed using a two-suction approach. To eliminate adherent adenoma and reduce blood loss, particularly from the cavernous sinus, physiological serum irrigation was employed to conclude the surgery. If no CSF (cerebrospinal fluid) leakage had been detected, a fat graft was sufficient. In instances of intraoperative CSF leakage, a multilayer closure with fascia lata, nasoseptal flap, fat graft and fibrin sealant was performed.
RESULTS
Without undergoing any medical or Gamma Knife radiosurgery prior to it, every patient received pure endoscopic pituitary operations.
Demographic Data, Signs and Symptoms of the Patients
There were 89 cases investigated. The patient cohort included 57 (64%) male patients and 32 (36%) female individuals (mean age of 39 years, the age range between 15 to 72 years). The average follow-up length was 12 months [Table 1].
The primary complaint among patients was the signs and symptoms of acromegaly. Visual field abnormalities were present in 20 patients (22.5%). Postoperatively, visual field improvements were observed in 16 patients (80%); of these, four patients (25%) experienced full recovery, ten patients (62.5%) had partial recovery, and two patients (12.5%) showed no significant changes.
Preoperative endocrinological deficiencies were noted in 16 out of 89 patients (17.9%), including hypogonadism in nine patients (10.1%), hypothyroidism in three patients (3.4%) and adrenal insufficiency in four patients (4.5%) [Table 2].
Remission According to Preoperative Data
Of the 82 primary instances, remission had been attained in 67 patients (81.7%). Three (42.9%) of the seven patients who had undergone prior procedures at other hospitals experienced remission following their procedure in our department.
Overall, remission was attained in 70 out of 89 patients (78.7%). This study included 78 patients with macroadenomas (87.6%) and 11 with microadenomas (12.4%). Remission was confirmed in 61 of the 78 macroadenoma cases (78.2%) and 9 of the 11 microadenoma cases (81.8%). For those who did not achieve remission, tumour volume ranged from 270 to 30,258 mm3 (mean, 3,838.72 mm3). The volume of the tumour ranged from 80 to 17,374 mm3 in the remission group (mean, 2,187.46 mm3).
Cavernous sinus invasion (Knosp grades III to IV) was present in 18 patients (20.2%). Among these, remission was achieved in seven patients (38.9%). Bilateral cavernous sinus invasion occurred in 3 out of the 18 patients (16.7%), with no remission seen in all of them. Compared with non-invasive tumours (88.7% in 63 out of 71 cases), tumours with cavernous sinus invasion had a lower remission rate (38.9%). However, the remission rate for non-invasive macroadenomas had been 81.8% (9 out of 11) versus 88.7% (63/71) for microadenomas.
Preoperative GH levels were categorised as follows: < 20 ng/mL in 64 patients, 20–40 in 14 patients, 40–60 in 5 patients, as well as > 60 ng/mL in 6 patients. Remission rates were 87.5% (56 out of 64) for GH levels < 20 ng/mL, 64.3% (9 out of 14) for levels between 20 and 40 ng/mL, 60% (3 out of 5) for levels between 40 and 60ng/mL and 33.3% (2 out of 6) for levels > 60 ng/mL [Table 3].
C) Remission According to Postoperative data
In 79 of the 89 patients, total resection had been accomplished (88.8%). Among the 79 patients, 67 (84.8%) satisfied the 2010 consensus criteria for remission. The excision group with extra-pseudocapsular involvement comprised 74 macroadenomas and 8 microadenomas. All patients with microadenomas (100%) attained remission, whereas 60 out of 74 patients (81.1%) with macroadenomas achieved remission. Overall, remission had been attained in 68 out of 82 individuals (82.4%) who underwent pseudocapsular resection [Table 3].
Within the first 72 hours postoperatively, GH levels had been under 1 ng/mL in 46 patients (51.7%) as well as under 2.5 ng/mL in 62 patients (69.7%). Mean postoperative GH levels were significantly lesser in remission patients in contrast to non-remission patients at all measured intervals (within 72 hours and at three and six months), as assessed by a t-test (P < 0.001) [Table 4, Figure 3].
Remission, based on age-related IGF values, was observed in 45 patients (50.6%) at one month, 65 patients (73.03%) at three months and 70 patients (78.7%) at six months postoperatively. A statistical examination of every patient who experienced remission over the course of the follow-up period revealed that a drop in IGF levels of more than 50% in the first month was a predictor of remission [Figure 4 and Figure 5].
D) Follow-Up of Non-Remission Groups
Of the 19 patients who failed to achieve remission, three (15.8%) did not attend further follow-up tests. Twelve (75%) of the 16 patients who did not achieve remission during the follow-up period underwent additional medical therapy, and four (33.3%) of these patients went on to achieve remission. Additionally, four patients underwent Gamma Knife surgery.
E) Statistical Results and Predictive Cut-Off Values
To evaluate the variables connected to remission, the Pearson correlation test was utilised. There was a significant positive connection (P < 0.001) among pseudocapsule resection as well as remission. However, there was a significant negative connection (P < 0.001) found between the cavernous sinus invasion along remission. Furthermore, a moderately positive connection (P < 0.001) was observed among the resection rate and remission. There was a weak but significant negative relationship (P = 0.017) between the size of the adenoma (macroadenoma vs. microadenoma) and remission. Tumour volume also showed a weak but significant negative correlation with remission (P < 0.001).
F) Complications
In this series, there were 11 complications out of 89 patients (12.3%). These complications were categorised into two groups: endocrinologic and anatomic. Endocrinologic complications occurred in six patients (6.7%), including temporary diabetes insipidus in two patients (2.2%) and hypogonadism, pituitary insufficiency and adrenal insufficiency in one patient each (1.1%). Anatomic complications were observed in five patients (5.6%), consisting of CSF leakage and epistaxis in two patients (2.2%) and a sellar hematoma in one patient (1.1%). No mortality occurred during the study period [Table 5].
| Gender | Number (percentage) |
|---|---|
| Male | 57 (64%) |
| Female | 32 (36%) |
| Age | Value (years) |
| Mean | 39 |
| Range | 15 to 72 |
| Follow up (months) | 12 months |
| Preoperative other endocrine deficiencies | 16(17.9%) |
| Hypogonadism | 9(10.1%) |
| Hypothyroidism | 3 (3.4%) |
| Adrenal insufficiency | 4 (4.5%) |
| Received remission | No remission | Total | P value | |
|---|---|---|---|---|
| Total | 70 (78.7%) | 19 (21.3%) | 89 | |
| Macroadenoma | 61 (78.2%) | 17 | 78 (87.6) | <0.017 |
| Microadenoma | 9(81.8%) | 2 | 11 (12.4) | |
| Cavernous sinus invasion | 7 (38.9 %) | 11 | 18 | <0.001 |
| B/L | 0 | 3 | 3 | |
| Pseudo capsular resection | 68 (82.9%) | 14 | 82 (92.1%) | <0.001 |
| Macroadenomas | 60 (81.1%) | 14 | 74 | |
| Microadenoma | 8 (100%) | 0 | 8 | |
| Preoperative GH levels (ng/ml) | ||||
| 0-20 | 56 (87.5%) | 8 | 64 (72%) | |
| 20-40 | 9 (64.3%) | 5 | 14 (15.7%) | |
| 40-60 | 3 (60%) | 2 | 5 (5.6%) | |
| >60 | 2 (33.3%) | 4 | 6 (6.7%) | |
| Primary cases | 67 (81.7%) | 15 (18.3%) | 82 (92.1%) | |
| Operated outside | 3 (42.9%) | 4(57.1%) | 7 | |
| Macroadenoma | 3 | 4 | 7 | |
| Microadenoma | 0 | 0 | 0 | |
| Volume (mm3) | 2187 (mean) | 3838 (mean) | <0.001 | |
| Resection rate | ||||
| Sub total | 3 (30%) | 7 | 10 (11.2%) | <0.001 |
| Total | 67 (84.8%) | 12 | 79 (88.8%) |
GH: Growth hormone, B/L: Bilateral
| Time | Mean GH values | P value | |
|---|---|---|---|
| No remission | Remission | ||
| Preoperative | 32.8 | 14.3 | P< 0.001 |
| Postoperative (72 hours) | 3.8 | 0.7 | P< 0.001 |
| Postoperative (3rd month) | 4.2 | 0.8 | P< 0.001 |
| Postoperative (6th month) | 4.3 | 0.8 | P< 0.001 |
GH: Growth hormone
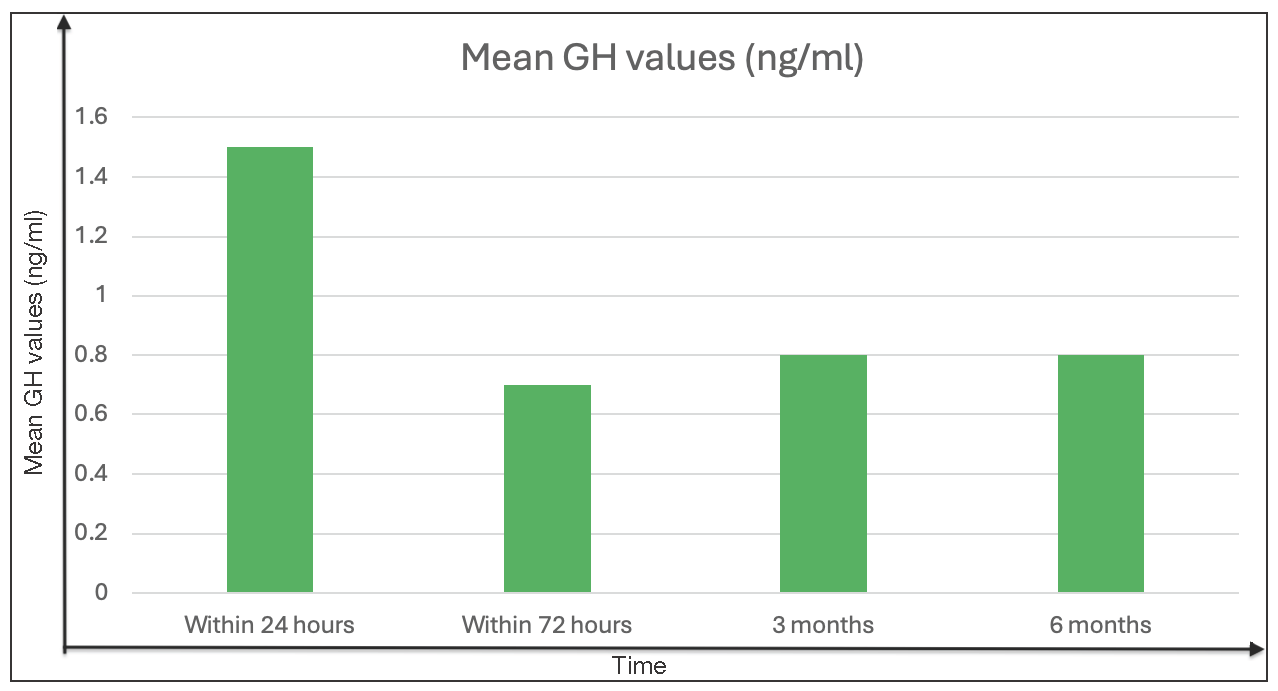
- Postoperative mean growth hormone values (ng/ml) within 72 hours on the third and sixth month in patients with remission (original).
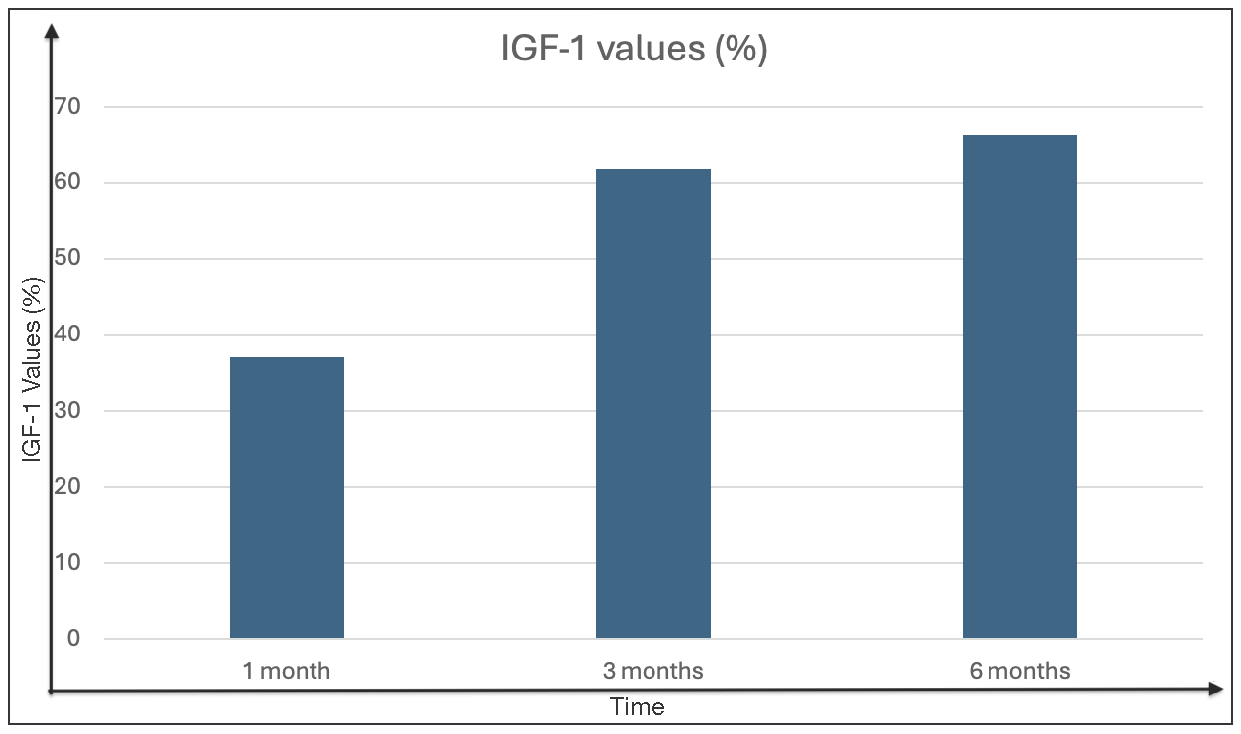
- Postoperative percentage of patients in remission according to age-related IGF values (original).
- Postoperative complications (original).
| Complications | Number (percentage) |
|---|---|
| Endocrine | 6 (6.7%) |
| Temporal D.I. | 2 (2.2) |
| Hypogonadism | 1 (1.1) |
| Pituitary Insufficiency | 1 (1.1) |
| Hypothyrdoidism | 1 (1.1) |
| Adrenal insufficiency | 1 (1.1) |
| Anatomical | 5 (5.6%) |
| CSF leak | 2 (2.2) |
| Epistaxis | 2 (2.2) |
| Sellar hematoma | 1 (1.1) |
D.I.: Diabetes insipidus, CSF: Cerebro spinal fluid
DISCUSSION
Surgical intervention is often recommended as the initial treatment for GH-secreting tumours. For patients who do not achieve remission through surgery alone, additional treatments such as radiation or medication may be necessary.[40] The endoscopic surgical method is favoured for several reasons, including less damage to the nasal area, better visibility during surgery, more comfort for the patient and a higher success rate in completely removing the tumour.[8,26,31,33]
Studies show a wide range of remission rates after surgery, from as low as 31.9% to as high as 84.6%.[8,16,20,40-50] A comprehensive analysis by Starnoni et al., which reviewed 13 studies involving 1105 patients, found an average remission rate of 54.8%.[47]
Research has pinpointed several factors that can predict the likelihood of remission before surgery. These factors include the size and volume of the tumour, levels of GH as well as IGF-1 and the tumour invasiveness extent.[9,13,27,35,36,40,51,52] In our research, we classified treatment outcomes based on both before and after-surgery findings. We looked at the type of tumour (microadenoma vs macroadenoma), its volume and GH levels before surgery to determine whether the tumour had invaded the cavernous sinus, the presence of extra-pseudocapsular dissection, GH levels after surgery and the rate of tumour resection. By classifying these outcomes, we aimed to understand better what influences remission rates.
Overall remission
The effectiveness of surgical treatments for acromegaly can be affected by various elements,[52] notably the tumour’s size. It’s generally observed that the success of surgery varies with the size of the tumour, with smaller tumours (microadenomas) usually having a better chance of remission compared to larger ones (macroadenomas).[47] Despite this, research conducted by Wang et al. indicated that the remission rates for large tumours, including macroadenomas and even giant adenomas, did not significantly differ from those for microadenomas.[52]
In our research, we achieved remission in 70 out of 89 patients (78.7%); breaking it down, 61 out of 78 patients with macroadenomas (78.2%) and 9 out of 11 patients with microadenomas (81.8%) reached remission, indicating promising outcomes when compared to previous studies [Table 6].
| ENDOSCOPIC series | Remission Rate | Total cases (macro/micro) | ||
|---|---|---|---|---|
| Overall | Macroadenoma | Microadenoma | ||
| Our Series | 79% | 78% | 82% | 89 (78/11) |
| Guo et al., 2022[53] | 81% | 145 | ||
| Jane et al., 2011[28] | 70% | 61% | 100% | 60 (46/14) |
| Chhabra et al., 2022[54] | 29% | 28 (28/0) | ||
| Taghvaei et al., 2018[55] | 63% | 61% | 75% | 68 (52/16) |
| Babu et al., 2017[56] | 69% | 67% | 74% | 58 (37/21) |
| Anik et al., 2017[57] | 68% | 63% | 81% | 401 (294/107) |
| Fathalla et al., 2014[42] | 35% | 20 (18/2) | ||
| Hazer et al., 2013[8] | 62% | 61% | 63% | 214 (163/5) |
| Shin et al., 2012[51] | 51% | 51 (45/6) | ||
| Hofstetter et al., 2010[43] | 38% | 24 (40/4) | ||
| Gondim et al., 2010[27] | 75% | 72% | 86% | 67 (53/14) |
| Campbell et al., 2010[58] | 58% | 55% | 75% | 26 (22/4) |
| Yano et al, 2009[30] | 71% | |||
| Dehdashti et al., 2008[59] | 71% | 65% | 83% | 34 (26/8) |
| Frank et al., 2006[60] | 70% | 65% | 83% | 83 (59/24) |
Our statistical analysis identified a meaningful, though inverse, relationship between the size of the adenoma and the likelihood of remission. Additionally, there was a slight yet statistically significant inverse relationship between the volume of the tumour and remission rates.
Giant adenomas pose a distinct challenge, often complicating complete removal and achieving remission significantly. Shimon et al. pointed out that among 762 acromegaly patients, 34 had giant adenomas (4.5%),[61] underlining the necessity for aggressive, multifaceted treatment strategies to reach biochemical remission and improve management outcomes. Such adenomas typically exhibit invasive growth beyond the sellar region, are tough to remove surgically and show limited response to pharmacological treatments.[61] In our study, we included two patients with giant adenomas, but unfortunately, neither achieved remission.
Cavernous sinus invasion
Cavernous sinus invasion is a critical factor in GH-secreting adenomas that fail to achieve remission. According to recent research, there is an increase in the incidence of pituitary adenomas causing cavernous sinus invasion.[22,62,60] For example, according to Nishioka et al., 69.1% of the 150 patients had remission after a cavernous sinus invasion in 55 out of the 150 patients (36.7%).[44] Nonetheless, remission was unattainable in any of the four individuals exhibiting grade IV cavernous sinus invasion.
In our investigation, remission was attained in 7 (38.9%) of the 18 patients (20.2%) with Knosp grade III/IV cavernous sinus invasion. According to our statistical research, the most significant variable influencing remission rates is cavernous sinus invasion.
When performing endoscopic procedures on the cavernous sinus, the lateral and medial pathways are essential. A recent clinical series indicates that pituitary adenomas frequently infiltrate the cavernous sinus through the medial corridor.[62,63] Pituitary adenomas establish access routes through these corridors, subsequently employed as surgical procedures. For lesions involving the cavernous sinus, we extended standard and contralateral surgical approaches.
Preoperative GH levels
Lower GH levels before surgery are connected along with an augmented probability of reaching biochemical remission. For example, research by Wilson indicated that patients with lower levels of GH prior to surgery have higher rates of remission.[14] Sarkar, et al. found that individuals with non-invasive, smaller tumours and lower GH levels before surgery tend to have a better chance of remission.[23] This study categorised patients based on their GH levels before surgery to assess whether these levels could serve as predictors for remission. It was found that 87.5% of individuals with GH levels below 20 ng/mL before surgery achieved remission; in contrast, only 33.3% of those had GH levels exceeding 60 ng/mL. The disparity in remission rates between these groups was statistically significant.
Postoperative GH levels
The measurement of levels of GH subsequent to an oral glucose tolerance test, performed 12 weeks or more post-surgery, is deemed a key predictor for determining whether acromegaly patients are heading towards remission.[5] However, the accuracy of random GH level assessments immediately after surgery is more ambiguous. Research by Dutta et al. into random GH level checks within the initial five days following transsphenoidal pituitary surgery as an indicator of enduring remission revealed that early post-surgery GH evaluations might be beneficial in forecasting long-term remission.[64] Specifically, a GH concentration lower than 1.5 ng/mL six hours postoperation was associated with remission in 2/3 of the cases. Hazer et al. also highlighted in their research that random GH concentrations < 2.33 ng/mL on the first-day post-surgery, coupled with a reduction exceeding 50% in the IGF-I levels after the first month, function as indicators of remission prediction.[8]
The categorisation of preoperative IGF values into abnormal or normal was done based on the age range of the patients. A decline of > 50% in IGF-I levels in the month following surgery suggests a move towards remission. According to Kim et al., who tracked GH levels at set intervals across 194 post-surgical patients, the most accurate predictor of long-term remission was the GH value on the first day postoperation.[50] A GH level lower than 1 ng/mL indicated an 88.6–92.1% likelihood of remission. In our investigation, 46 patients exhibited GH levels under 1 ng/mL within the initial 72 hours post-surgery.
It’s crucial to monitor IGF-I concentrations and GH levels, adjusted for age, to effectively track the response to treatment and the progression of the disease.[27] Although GH levels can be assessed during the first week following surgery, the levels of IGF-I are recommended to be assessed no sooner than 12 weeks since they stabilise around three months and may vary up to a year.[5] Sometimes, GH and IGF-I levels might show conflicting results, such as elevated IGF-I alongside normal GH levels or the reverse. In such scenarios, the clinical symptoms indicating excessive GH should be scrutinised. If clinical improvement is observed, it is advised to continue regular hormonal evaluations and follow-up visits every three to six months without rushing into further medical interventions.[65]
Extra-pseudocapsular dissection
In previous studies, it was found that using a pseudocapsule-based extracapsular excision approach can lead to enhanced postoperative outcomes, for example, higher remission rates as well as lower recurrence rates.[2,63,66,67] After confirming the presence of a pseudocapsule endoscopically, extra-pseudocapsular dissection was performed while maintaining the pseudocapsule’s integrity.[68]
In this study, the pseudocapsular group mainly comprised 74 macroadenomas and 8 microadenomas. Detecting pseudocapsules in microadenomas using endoscopy is more challenging than in macroadenomas, possibly due to the limited detailed visualisation provided by the endoscopic technique in smaller areas compared to the microscopic technique. In the current investigations, extra-pseudocapsular dissection was carried out in 82 individuals, leading to remission in 68 patients (82.9%). Among the extra-pseudocapsular resection as well as remission, a statistically significant relationship had been identified, with remission not observed in most cases where extra-pseudocapsular resection could not be achieved.
Postoperative resection rates
In patients with GH-secreting adenomas, the surgical method is critical to obtaining total resection and postoperative remission. Extracapsular dissection can significantly increase the rates of total resection as well as remission.[68] Therefore, an aggressive extracapsular method is preferred for these adenomas. Nevertheless, this method can occasionally result in complications, including CSF leakage along with suprasellar cistern rupture. In these instances, we often execute closure in several layers, and lumbar drainage may be utilised for four to five days to address elevated pressure CSF leak.
A previous study found no significant difference in pituitary insufficiency rates between patients who underwent an aggressive approach and those who did not.[68] Despite this, the potential for pituitary insufficiency is considered an acceptable risk to achieve remission, with replacement therapy applied as needed.
Achieving remission in recurrent cases is more complex and often requires the use of neuronavigation to verify anatomical landmarks. In our experience, intraoperative observations of various macroscopic tumour characteristics, such as white soft adenomas, fibrotic membranes, hard shells and rarely calcified adenomas, necessitate different strategies. If postoperative clinical and biochemical evaluations indicate persistent disease, medical therapy is used to control the condition.[5]
Medical treatment aims to normalise IGF-I levels and GH hypersecretion, control or reduce tumour size and address mortality and morbidity rates.[1] Cabergoline may be employed as early adjuvant therapy for patients exhibiting mild symptoms and moderately raised serum IGF-1 levels. In more severe instances, initial therapy with a pegvisomant or an SRL (somatostatin receptor ligand) is suggested.[1,5] Combination therapy utilising cabergoline, SRLs or pegvisomant is utilised for uncontrolled disease. Pituitary imaging in conjunction with GH and IGF-1 level tests is employed to evaluate the efficacy of treatment.
A current investigation highlighted the importance of understanding the genetic landscape and receptor profiles of different tumours.[69] Understanding the molecular abnormalities that cause pituitary tumourigenesis is essential for patient care since it helps with early intervention, concomitant condition screening, genetic counselling and selecting the best course of treatment, including surgery and medicine.[70]
CONCLUSION
With a high incidence of remission and few side effects, the endoscopic endonasal transsphenoidal procedure is a safe and efficient way to treat adenomas in patients with acromegaly. Remission is predicted by factors that include GH levels, tumour size, cavernous sinus invasion as well as total resection. The careful elimination of minute remnants should be the main surgical goal. Despite available treatments, including surgery, medical therapy and radiotherapy, many patients still experience inadequate disease control.
Ethical approval
Institutional Review Board approval is not required for this study is retrospective in nature and involved the review of pre-existing, anonymized patient data. According to our institutional policy, ethical approval is not required for studies that do not involve direct patient interaction or any intervention affecting patient care. Furthermore, all data were collected and analyzed in compliance with institutional and national guidelines to ensure confidentiality and privacy.
Declaration of patients consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
REFERENCES
- The Modern Criteria for Medical Management of Acromegaly. Prog Mol Biol Transl Sci. 2016;138:63-83.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of using the Histological Pseudocapsule as a Surgical Capsule in Cushing Disease. J Neurosurg. 2009;111:531-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acromegaly Pathogenesis and Treatment. J Clin Invest. 2009;119:3189-202.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Consensus on the Diagnosis and Treatment of Acromegaly Complications. Pituitary. 2013;16:294-302.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Acromegaly: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:3933-51.
- [CrossRef] [PubMed] [Google Scholar]
- Transsphenoidal Microsurgery for Newly Diagnosed Acromegaly: A Personal View After more than 1,000 Operations. Neuroendocrinology. 2006;83:230-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, Hormonal and Magnetic Resonance Imaging (MRI) Predictors of Transsphenoidal Surgery Outcome in Acromegaly. Eur J Endocrinol. 2004;150:763-71.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Acromegaly by Endoscopic Transsphenoidal Surgery: Surgical Experience in 214 Cases and Cure Rates According to Current Consensus Criteria. J Neurosurg. 2013;119:1467-77.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of Long-term Remission of Acromegaly Following Surgery. J Neurosurg. 2003;98:719-24.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary Surgery. Endocrinol Metab Clin North Am. 1999;28:119-31.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for Acromegaly Management. J Clin Endocrinol Metab. 2002;87:4054-8.
- [CrossRef] [PubMed] [Google Scholar]
- Current Treatment Guidelines for Acromegaly. J Clin Endocrinol Metab. 1998;83:2646-52.
- [CrossRef] [PubMed] [Google Scholar]
- Pituitary Tumors: An Endocrinological and Neurosurgical Challenge. Clin Neurosurg. 1992;39:114-22.
- [PubMed] [Google Scholar]
- Surgical Management of Pituitary Tumors. J Clin Endocrinol Metab. 1997;82:2381-5.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical Results of Growth Hormone-secreting Pituitary Adenoma. J Korean Neurosurg Soc. 2009;45:271-4.
- [CrossRef] [PubMed] [Google Scholar]
- Factors Influencing the Outcome of Microsurgical Transsphenoidal Surgery for Pituitary Adenomas: A Study on 184 Patients. Hormones (Athens). 2013;12:254-64.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term Outcome and Mortality After Transsphenoidal Adenomectomy for Acromegaly. Clin Endocrinol (Oxf). 2003;58:86-91.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical Management of GH-secreting Pituitary Adenomas: An Outcome Study using Modern Remission Criteria. J Clin Endocrinol Metab. 2001;86:4072-7.
- [CrossRef] [PubMed] [Google Scholar]
- Editorial: Unresolved Issues: Radiosurgery Versus Radiation Therapy; Medical Suppression of Growth Hormone Production During Radiosurgery; and Endoscopic Surgery Versus Microscopic Surgery. Neurosurg Focus. 2010;29:E16.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic vs Microsurgical Transsphenoidal Surgery for Acromegaly: Outcomes in a Concurrent Series of Patients using Modern Criteria for Remission. J Clin Endocrinol Metab. 2013;98:3190-8.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Pituitary Surgery: A Systematic Review and Meta-analysis. J Neurosurg. 2009;111:545-54.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Transsphenoidal Approach for Pituitary Adenomas Invading the Cavernous Sinus. J Neurosurg. 2010;112:99-107.
- [CrossRef] [PubMed] [Google Scholar]
- Endocrinological Outcomes Following Endoscopic and Microscopic Transsphenoidal Surgery in 113 patients with Acromegaly. Clin Neurol Neurosurg. 2014;126:190-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Versus Microsurgical Transsphenoidal Approach for Growth Hormone-secreting Pituitary Adenomas-Systematic Review and Meta-analysis. World Neurosurg. 2017;97:398-406.
- [CrossRef] [PubMed] [Google Scholar]
- A Consensus on Criteria for Cure of Acromegaly. J Clin Endocrinol Metab. 2010;95:3141-8.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of the Outcome of Surgical Treatment in Acromegaly and the Value of the Mean Growth Hormone Day Curve in Assessing Postoperative Disease Activity. J Clin Endocrinol Metab. 2001;86:1645-52.
- [CrossRef] [PubMed] [Google Scholar]
- Pure Endoscopic Transsphenoidal Surgery for Treatment of Acromegaly: Results of 67 Cases Treated in a Pituitary Center. Neurosurg Focus. 2010;29:E7.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Transsphenoidal Surgery for Acromegaly: Remission using Modern Criteria, Complications, and Predictors of Outcome. J Clin Endocrinol Metab. 2011;96:2732-40.
- [CrossRef] [PubMed] [Google Scholar]
- The Outcome of Surgery in 668 Patients with Acromegaly using Current Criteria of Biochemical ‘Cure’. Eur J Endocrinol. 2005;152:379-87.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Transsphenoidal Approach through the Bilateral Nostrils for Pituitary Adenomas. Neurol Med Chir (Tokyo). 2009;49:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Factors Determining the Long-term Outcome of Surgery for Acromegaly. QJM. 1994;87:617-23.
- [PubMed] [Google Scholar]
- Outcome of Transsphenoidal Surgery for Acromegaly using Strict Criteria for Surgical Cure. Clin Endocrinol (Oxf). 1996;45:407-13.
- [CrossRef] [PubMed] [Google Scholar]
- Transsphenoidal Surgery for Acromegaly: Endocrinological Follow-up of 98 Patients. Neurosurgery. 2001;48:1239-43. discussion 1244–5
- [CrossRef] [PubMed] [Google Scholar]
- [Outcome of Surgical Treatment for Acromegaly Performed by a Single Neurosurgeon and Cumulative Meta-analysis] Arq Bras Endocrinol Metabol. 2006;50:884-92.
- [CrossRef] [PubMed] [Google Scholar]
- Psuedo-capsule Based Extracapsular Resection of Pituitary Adenoma. Egypt J Neurosurg. 2015;30:113-6.
- [Google Scholar]
- Surgical Removal of Growth Hormone-secreting Pituitary Adenomas with Intensive Microsurgical Pseudocapsule Resection Results in Complete Remission of Acromegaly. Neurosurg Rev. 2005;28:201-8.
- [CrossRef] [PubMed] [Google Scholar]
- Invasion of the Cavernous Sinus Space in Pituitary Adenomas: Endoscopic Verification and its Correlation with an MRI-based Classification. J Neurosurg. 2015;122:803-11.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Modified Transseptal Transsphenoidal Approach for Maximal Preservation of Sinonasal Quality of Life and Olfaction. World Neurosurg. 2016;87:162-9.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility of Endoscopic Endonasal Approach for Recurrent Pituitary Adenomas After Microscopic Trans-sphenoidal Approach. J Korean Neurosurg Soc. 2013;54:317-22.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Transsphenoidal Surgery for Acromegaly: Predicting Remission with Early Postoperative Growth Hormone Assays. Acta Neurochir (Wien). 2014;156:1379-87. discussion 1387
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of Multimodal Therapy in Operated Acromegalic Patients, a Study in 115 Patients. Clin Endocrinol (Oxf). 2013;78:263-70.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Versus Microscopic Approach for Surgical Treatment of Acromegaly. Neurosurg Rev. 2015;38:541-8. discussion 548–9
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Transsphenoidal Surgery for Growth Hormone-secreting Pituitary Adenomas. Neurosurg Focus. 2010;29:E6.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressive Transsphenoidal Resection of Tumors Invading the Cavernous Sinus in Patients with Acromegaly: Predictive Factors, Strategies, and Outcomes. J Neurosurg. 2014;121:505-10.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Approach for Pituitary Adenomas: A Series of 555 Patients. Pituitary. 2014;17:307-19.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Approach for Growth Hormone Secreting Pituitary Adenomas: Outcomes in 53 Patients using 2010 Consensus Criteria for Remission. Pituitary. 2013;16:435-44.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical Treatment of Acromegaly According to the 2010 Remission Criteria: Systematic Review and Meta-analysis. Acta Neurochir (Wien). 2016;158:2109-21.
- [CrossRef] [PubMed] [Google Scholar]
- Factors Associated with biochemical Remission After Microscopic Transsphenoidal Surgery for Acromegaly. J Neurol Surg B Skull Base. 2014;75:47-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endoscopic Endonasal Transsphenoidal Treatment for Acromegaly: 2010 Consensus Criteria for Remission and Predictors of Outcomes. Turk Neurosurg. 2014;24:906-12.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting Long-term Remission by Measuring Immediate Postoperative Growth Hormone Levels and Oral Glucose Tolerance Test in Acromegaly. Neurosurgery. 2012;70:1106-13. discussion 1113
- [CrossRef] [PubMed] [Google Scholar]
- Transsphenoidal Surgery for Growth Hormone-secreting Pituitary Adenomas in 130 Patients. World Neurosurg. 2014;81:125-30.
- [CrossRef] [PubMed] [Google Scholar]
- Acromegaly Surgery in Manchester Revisited--the Impact of Reducing Surgeon Numbers and the 2010 Consensus Guidelines for Disease Remission. Clin Endocrinol (Oxf). 2012;76:399-406.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of Immediate and Long-term Remission After Initial Transsphenoidal Surgery for Acromegaly and Outcome Patterns During Follow-up: A Longitudinal Study on 659 Patients. J Neurosurg. 2022;137:618-28.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes in Pituitary Adenoma Causing Acromegaly Following Endoscopic Endonasal Transsphenoidal Surgery. J Neurosci Rural Pract. 2022;13:696-704.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Endoscopic Endonasal Approach to the Growth Hormone-secreting Pituitary Adenomas: Endocrinologic Outcome in 68 Patients. World Neurosurg. 2018;117:e259-68.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term Endocrine Outcomes Following Endoscopic Endonasal Transsphenoidal Surgery for Acromegaly and Associated Prognostic Factors. Neurosurgery. 2017;81:357-66.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Transsphenoidal Approach for Acromegaly with Remission Rates in 401 Patients: 2010 Consensus Criteria. World Neurosurg. 2017;108:278-90.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes After a Purely Endoscopic Transsphenoidal Resection of Growth Hormone-secreting Pituitary Adenomas. Neurosurg Focus. 2010;29:E5.
- [CrossRef] [PubMed] [Google Scholar]
- Pure Endoscopic Endonasal Approach for Pituitary Adenomas: Early Surgical Results in 200 Patients and Comparison with Previous Microsurgical Series. Neurosurgery. 2008;62:1006-15. discussion 1015–7
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Endonasal Cavernous Sinus Surgery, with Special Reference to Pituitary Adenomas. Front Horm Res. 2006;34:64-82.
- [CrossRef] [PubMed] [Google Scholar]
- Giant GH-secreting Pituitary Adenomas: Management of Rare and Aggressive Pituitary Tumors. Eur J Endocrinol. 2015;172:707-13.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Transnasal Approach to the Cavernous Sinus Versus Transcranial Route: Anatomic Study. Neurosurgery. 2005;56:379-89. discussion 379–89
- [CrossRef] [PubMed] [Google Scholar]
- Microsurgical Anatomy of Membranous Layers of the Pituitary Gland and the Expression of Extracellular Matrix Collagenous Proteins. Acta Neurochir (Wien). 2011;153:2435-43. discussion 2443
- [CrossRef] [PubMed] [Google Scholar]
- Can Immediate Postoperative Random Growth Hormone Levels Predict Long-term Cure in Patients with Acromegaly? Neurol India. 2016;64:252-8.
- [CrossRef] [PubMed] [Google Scholar]
- Discordance between Growth Hormone and Insulin-like Growth Factor-1 after Pituitary Surgery for Acromegaly: A Stepwise Approach and Management. Pituitary. 2015;18:48-59.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a Histological Pseudocapsule and its Use as a Surgical Capsule in the Excision of Pituitary Tumors. J Neurosurg. 2006;104:7-19.
- [CrossRef] [PubMed] [Google Scholar]
- Transsphenoidal Pseudocapsule-based Extracapsular Resection for Pituitary Adenomas. Acta Neurochir (Wien). 2011;153:799-806.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic Distinction Between Capsule and Pseudocapsule of Pituitary Adenomas. Acta Neurochir (Wien). 2013;155:1611-9. discussion 1619
- [CrossRef] [PubMed] [Google Scholar]
- The Genome-wide Mutational Landscape of Pituitary Adenomas. Cell Res. 2016;26:1255-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pituitary Gigantism: Update on Molecular Biology and Management. Curr Opin Endocrinol Diabetes Obes. 2016;23:72-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







